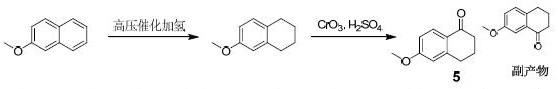
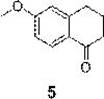
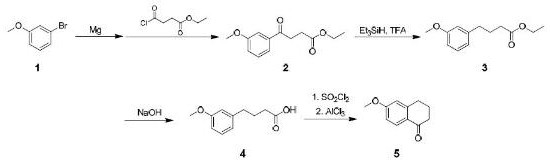
Synthesis method of 6-methoxy-1-tetralone
A synthesis method and tetralinone technology are applied in the field of preparation of substituted tetralinone, and can solve the problems of difficult separation and removal, high safety risk, low total yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthesis of embodiment 1 compound (2):
[0020] In the reaction bottle A, add 400g of toluene and 12.7g of magnesium flakes, fully replace with nitrogen, raise the temperature to 80°C, add 0.5mL of methyl iodide, after the reaction is initiated, dropwise add 3-bromoanisole (1) (100g) of toluene ( 1600g) solution, temperature control 60~100°C, dropwise addition is completed in about 2 hours; keep warm for 4 hours until the magnesium flakes disappear completely; cool to 0°C to obtain 3-methoxyphenylmagnesium bromide solution;
[0021] In the reaction bottle B, add 1000 g of toluene and 200 g of ethyl succinate monoacyl chloride, stir to dissolve, and cool to -50 ° C; dropwise add the 3-methoxyphenyl magnesium bromide solution obtained above, about 2 hours, control Temperature below -40°C; after adding, keep warm for 2 hours; quench the reaction solution into dilute sulfuric acid, separate layers, wash with water, wash with saturated sodium bicarbonate, wash with satu...
Embodiment 2
[0022] The synthesis of embodiment 2 compound (3):
[0023] In the reaction flask C, add all the crude compound (2) prepared in Example 1 and 600 g of dichloromethane to prepare a dichloromethane solution of compound 2;
[0024] In the reaction flask D, add 800g of dichloromethane and 200g of triethylsilane, cool down to 0°C, add 150g of trifluoroacetic acid dropwise; raise the temperature to 15~20°C, add the dichloromethane solution of compound 2 dropwise for about 1 hour, Control the temperature at 10-30°C; keep warm for 3 hours after addition; add 800 g of water dropwise, and stir for 2 hours; let stand to separate layers, wash with water, wash with saturated saline, and dry over anhydrous sodium sulfate; concentrate the organic phase to obtain the oily compound (3).
Embodiment 3
[0025] Synthesis of Example 3 Compound 4
[0026] In the reaction flask, add all the crude compound (3) obtained in Example 2 and 800 g of methanol, stir and dissolve; add 30 g of sodium hydroxide and 800 g of water; heat up to reflux, and reflux for 4 hours; after the reaction solution is concentrated to remove methanol, use Washed twice with ethyl acetate, then adjusted the pH to 2 with 4M hydrochloric acid, extracted three times with ethyl acetate, combined the organic phases, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 83 g of a yellow solid, namely compound (4)); The total mass yield of the first step reaction was 83%, and the HPLC purity was 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com