Green and environment-friendly method for synthesizing veratraldehyde
A green and environmentally friendly veratraldehyde technology, applied in chemical instruments and methods, condensation preparation of carbonyl compounds, physical/chemical process catalysts, etc., can solve the problem of difficult control of chlorination process, easy generation of monochlorine and trichlorine by-products, conversion The efficiency and purity are not high, so as to overcome the difficulty of catalyst separation, reduce the environmental protection and biochemical intensity, and avoid the effect of low production yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of green environment-friendly method for synthesizing veratraldehyde, comprises the following steps:
[0034] S1. Add vanillin, 1-methoxy-2,2,6,6-tetramethylpiperidine and cesium carbonate to toluene, heat up to 70° C., stir and keep warm for 8 hours;
[0035] The preparation method of the 1-methoxy-2,2,6,6-tetramethylpiperidine is:
[0036] Weigh 5.0 g of 2,2,6,6-tetramethylpiperidin-1-oxyl radical (CAS No.: 2564-83-2) and dissolve it in cold dimethyl sulfoxide solution, mix well Add 12.2g of ferrous sulfate heptahydrate, add 18mL of 30% hydrogen peroxide solution drop by drop under ice-water bath and stirring conditions, keep stirring for 1 hour after the addition is completed, and add 100mL of deionized water after the reaction is completed Dilute, then extract with ether, take the organic phase, wash with brine, dry over anhydrous sodium sulfate, concentrate, and purify by silica gel chromatography (500 mesh), eluting with ethyl acetate-hexane (v / v=1:19) , ...
Embodiment 2
[0040] A kind of green environment-friendly method for synthesizing veratraldehyde, comprises the following steps:
[0041] S1. Add vanillin, 1-methoxy-2,2,6,6-tetramethylpiperidine and barium hydroxide to toluene, heat up to 70°C, stir and keep warm for 8 hours;
[0042] The preparation method of the 1-methoxy-2,2,6,6-tetramethylpiperidine is the same as in Example 1;
[0043] The mass ratio of the toluene to the vanillin, the 1-methoxy-2,2,6,6-tetramethylpiperidine and the barium hydroxide is 10:1:1.2:0.6;
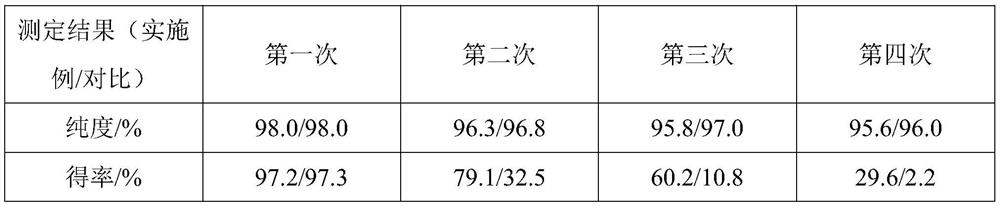
[0044] S2, after the stirring reaction is completed, the reaction system is washed with deionized water, the organic layer is collected, and the toluene is evaporated to obtain veratraldehyde. Through gas chromatography analysis, the purity of the reveratrol product is 97.5%, and the yield is 95.6%.
Embodiment 3
[0046] A kind of green environment-friendly method for synthesizing veratraldehyde, comprises the following steps:
[0047] S1. Add vanillin, 1-methoxy-2,2,6,6-tetramethylpiperidine and a porous carbon material loaded with cesium carbonate into toluene, heat up to 70° C., stir and keep warm for 8 hours;
[0048] The preparation method of the 1-methoxy-2,2,6,6-tetramethylpiperidine is the same as in Example 1;
[0049] The preparation method of the porous carbon material loaded with cesium carbonate is:
[0050] (1) Template preparation
[0051] Weigh 3g of P123 and dissolve it in 20mL of absolute ethanol, fully dissolve and mix, add 0.13g of cesium nitrate, mix again, add dropwise 6.2g of tetraethyl orthosilicate and 0.7mL of hydrochloric acid solution (1mol / L) while stirring , after the dropwise addition, let it stand, evaporate the solvent and perform heat preservation and heat treatment at 580-650 ° C to obtain a doped SBA template;
[0052] (2) Weigh 2.5g of glucose and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com