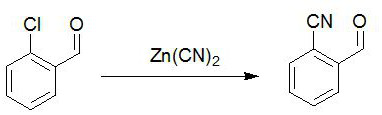
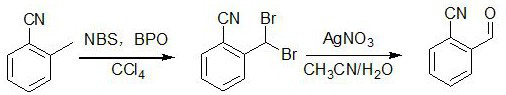
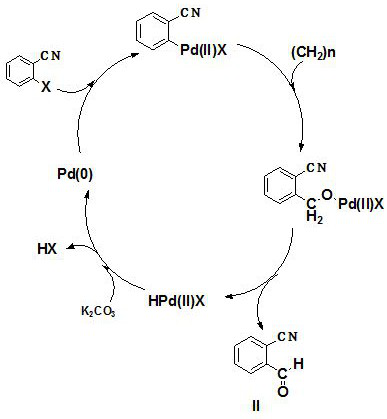
Preparation method of 2-cyanobenzaldehyde and derivatives thereof
A technology of derivatives and cyanobenzene, which is applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as low reaction yields, hidden safety hazards, and harsh reaction conditions, and achieve high reaction yields, high utilization rates, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: a kind of preparation method of 2-cyanobenzaldehyde
[0038] Add 600ml of toluene to a 1L three-necked reaction flask, start stirring, and then add Pd(TFA) 2 1.99g (0.006mol), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene 5.79g (0.01mol) and potassium carbonate 41.46g (0.30mol), 2-bromobenzonitrile 36.40 g (0.2 mol), paraformaldehyde 12.00 g (0.4 mol); the reaction mixture was heated to 100°C, and reacted for 12 hours. After the reaction is completed, cool down to room temperature and filter; the filtrate is extracted and washed with saturated saline for 3 times (the amount of saturated saline is 60ml / time), and 4.0g of activated carbon is added to the extracted and washed filtrate to decolorize at room temperature for 1.5h. Filter through the funnel, remove the activated carbon, and remove the toluene with a rotary evaporator to obtain crude 2-cyanobenzaldehyde as a brownish-yellow solid. The crude product is recrystallized with toluene and dried to obta...
Embodiment 2
[0040] Embodiment 2: a kind of preparation method of 2-cyanobenzaldehyde
[0041] Add 600ml of toluene to a 1L three-necked reaction flask, start stirring, and then add Pd(TFA) 2 0.66g (0.002mol), 1,2-bis(diphenylphosphine)ethane 1.59g (0.004mol) and potassium bicarbonate 20.02g (0.20mol), 2-chlorobenzonitrile 27.51g (0.2mol) , 6.00 g (0.2 mol) of paraformaldehyde; the reaction mixture was heated to 80° C., and kept for 6 hours. After the reaction is completed, cool down to room temperature and filter; the filtrate is extracted and washed twice with saturated saline (the amount of saturated saline is 50ml / time), and 3.0g of activated carbon is added to the extracted and washed filtrate to decolorize at room temperature for 1 hour. Filtrate, remove the activated carbon, and remove the toluene with a rotary evaporator to obtain crude 2-cyanobenzaldehyde as a brownish-yellow solid. The crude product is recrystallized with toluene and dried to obtain high-purity 2-cyanobenzaldehy...
Embodiment 3
[0043] Embodiment 3: a kind of preparation method of 2-cyanobenzaldehyde
[0044]Add 600ml of toluene to a 1L three-neck reaction flask, start stirring, and then add Pd(OAC) 2 2.25g (0.01mol), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene 11.57g (0.02mol) and potassium carbonate 55.28g (0.40mol), 2-bromobenzonitrile 36.40g (0.2mol), paraformaldehyde 18.0g (0.6mol); the reaction mixture was heated up to 110°C, and kept for 24h. After the reaction is completed, cool down to room temperature and filter; the filtrate is extracted and washed with saturated saline for 3 times (the amount of saturated saline is 80ml / time), and 6.0g of activated carbon is added to the extracted and washed filtrate to decolorize at room temperature for 2 hours. Filtrate, remove the activated carbon, and remove the toluene with a rotary evaporator to obtain crude 2-cyanobenzaldehyde as a brownish-yellow solid. The crude product is recrystallized with toluene and dried to obtain high-purity 2-cyanobenza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com