Fusion expression protein as well as preparation method and application thereof
A technology of fusion protein and expression vector, which is applied in the field of fusion expression protein and its preparation, can solve the problems of high cost, complex preparation method of composite quality control products, inconvenient operation, etc., achieve low cost, improve detection convenience, and good stability and accuracy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 synthetic target gene
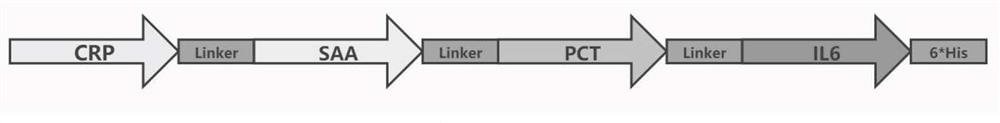
[0051] Query the complete CDS region of 4 genes in NCBI, among which, the nucleotide sequences of CRP gene, SAA gene, PCT gene and IL6 sequence are respectively as SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:3, SEQ ID NO: ID NO:4 shown.
[0052] Insert Hind III restriction endonuclease cutting site and Kozak sequence before CRP initiation codon ATG, wherein the nucleotide sequence of Hind III restriction endonuclease cutting site is as shown in SEQ ID NO:5 ( AAGCTT), the nucleotide sequence of Kozak is shown in SEQ ID NO: 6 (GCCACCATGG), the purpose of inserting the Kozak sequence among the present invention is to ensure that the target gene transcript can be effectively translated.
[0053] Insert the 6*His tag before the IL6 stop codon TAG, the nucleotide sequence is as shown in SEQ ID NO:7 (CATCATCATCATCATCAC), and insert the Xba I restriction site after the TAG, the nucleotide sequence is as SEQ ID NO:8 Shown (TCTAGA). At...
Embodiment 2
[0056] The construction of embodiment 2 recombinant vector
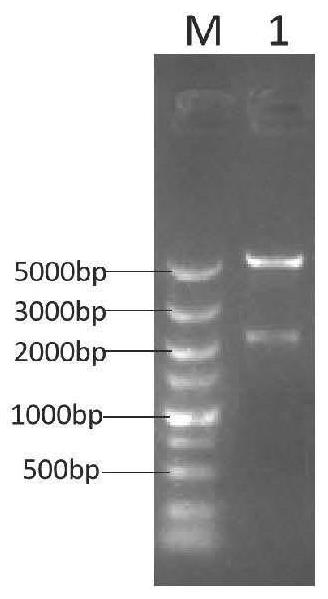
[0057] The target gene synthesized in Example 1 and the mammalian expression vector pcDNA3.1 were simultaneously digested with Hind III and Xba I restriction endonucleases for 30 min, and the enzyme digestion system is shown in Table 1 below. The digested products were detected by agarose gel electrophoresis and recovered for later use.
[0058] Table 1 enzyme digestion system
[0059] Reagent Volume / μL pcDNA3.1 / recombination target gene 3.6 / 2.6 10×Cut buffer 4 Hind III 1.5 Xba I 1.5 sterile water Make up to 40μL
[0060] The recovered target gene fragment was ligated with pcDNA3.1 at 6 times the mass ratio of the vector with T4 DNA ligase at 16°C overnight, and the ligated product was transformed into Top 10 competent cells, and plated on LB containing 50 μg / mL ampicillin Solid culture medium, cultured upside down in a constant temperature incubator at 37°C for 12-16 ...
Embodiment 3
[0062] Expression and identification of embodiment 3 fusion protein
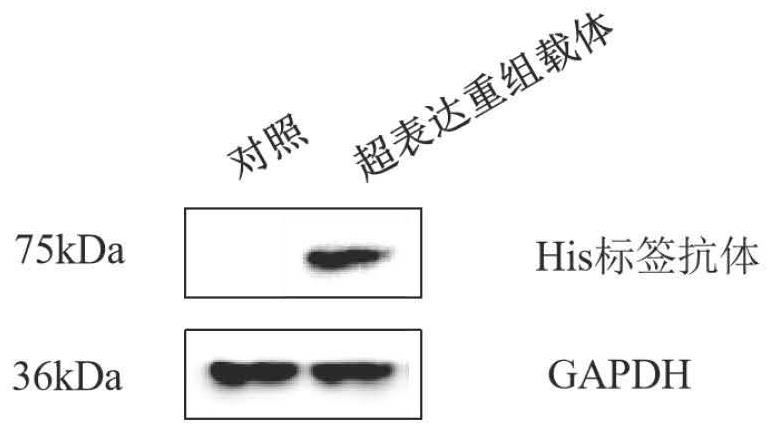
[0063] (1) After further culturing the recombinant vector successfully obtained in Example 2, use an endotoxin-free plasmid extraction kit to obtain a large number of recombinant plasmids for future use.
[0064] (2) Resuscitate HEK 293T cells, culture the cells after subculture until the cells grow to a density of about 70-80%, and then transfect.
[0065] (3) Dilute the plasmid and Lipofectamine 2000 transfection reagent in Opti-MEM and let it stand for 5 minutes. After mixing the plasmid and transfection reagent, let it stand for 30 minutes, add it evenly to the cell culture dish, and culture in the cell incubator.
[0066] (4) After the cells have been cultured for 72 hours, take out the cells from the incubator, wash the residual medium with PBS, lyse the cells with RIPA lysate (containing protease inhibitors and PMSF), scrape the cells with the thick part of a yellow pipette, and then suck out the liqu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com