Preparation method of recombinant Protein A protein and affinity chromatography medium
A protein and histidine technology, applied in the field of affinity chromatography, to achieve the effects of good alkaline stability, improved alkaline stability, and excellent IgG binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
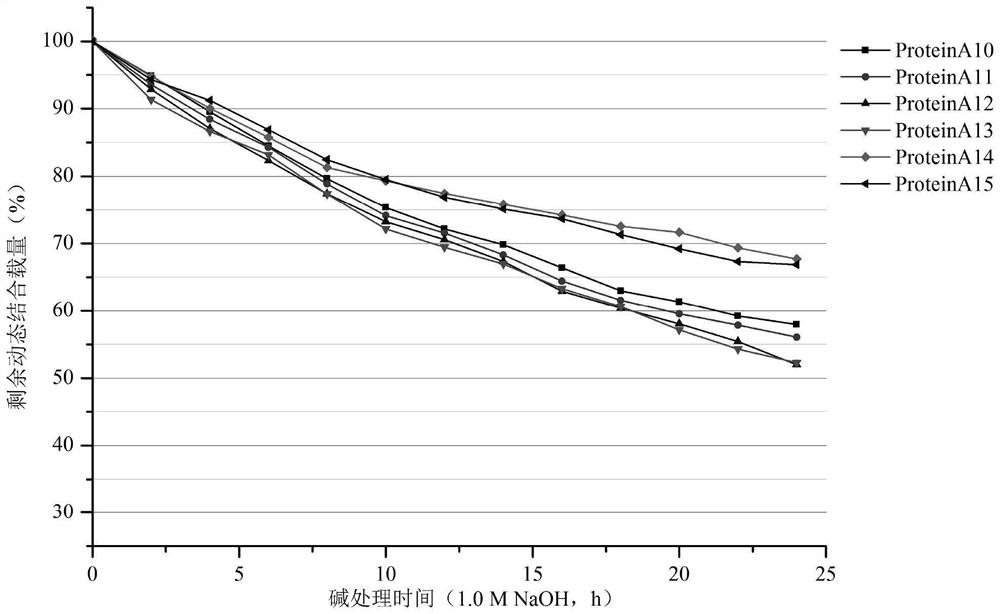
Image
Examples
Embodiment 1
[0107] 1. Protein mutagenesis of Protein A protein with mutations in B domain or Z domain
[0108] In order to improve the chemical stability of Protein A protein, there are more and more studies on modifying it by protein engineering to prepare recombinant Protein A protein as a ligand to improve the stability of affinity chromatography column alkali. Patent CN104804073B introduces mutations in the protein A protein amino acid sequence into leucine, arginine and lysine, and the recombinant protein A protein shows an enhanced binding ability to IgG compared with the natural protein A protein. In patent US6399750 B1, cysteine is mutated into Protein A protein, and the obtained recombinant Protein A protein is easy to immobilize on the surface of water-insoluble carrier, which has the advantages of high binding capacity and the ligand is not easy to fall off. Patent CN102365361A mutated into Protein A protein tyrosine and histidine, and found that compared with before mutation...
Embodiment 2
[0159] Example 2 Recombinant Protein A protein with D domain mutation
[0160] 1. Optimization of Protein A protein gene
[0161] Based on the technical scheme of the invention, based on the sequence of SEQ ID NO: 21, the asparagine at the 6th position of the amino acid sequence of the specified D domain has been mutated to be selected from glutamine, serine, histidine, lysine, arginine amino acid, leucine, tyrosine; the asparagine at the 9th position has been mutated to be selected from aspartic acid, glutamine, histidine, lysine; the glutamine at the 12th position has been mutated Mutated to be selected from lysine, leucine, serine, threonine, arginine; glutamic acid at position 18 has been mutated to be selected from histidine, lysine, leucine, serine, threonine Amino acid, arginine, the designed single-point amino acid mutation sequence is shown in SEQ ID NO:22~SEQ ID NO:43; at the same time, in a preferred embodiment, two site mutations are performed on the sequence o...
Embodiment 3
[0195] Example 3 Recombinant Protein A protein with A domain mutation
[0196] 1. Optimization of Protein A protein gene
[0197] The A domain sequence is defined by SEQ ID NO:64
[0198] Based on the technical scheme of the invention, amino acid sequences such as SEQ ID NO:65~SEQ ID NO:86 are designed and translated into nucleotide sequences and entrusted to a gene synthesis company for gene synthesis.
[0199] 2. Expression and purification of recombinant Protein A protein with A domain mutation
[0200] The synthesized amino acid sequence was transformed into Escherichia coli BL21(DE3) through the recombinant plasmid PET30a vector, and the fermentation culture was carried out through LB liquid medium. After fermentation, the substances in the periplasm are dispersed into the culture medium by thermal cracking and centrifuged to obtain the expression substance solution.
[0201] Purify the separated expression substance solution through the IgG chromatography medium, l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com