Modified blood purification membrane and preparation method thereof
A blood purification and modification technology, applied in the field of medicine, can solve the problems of reducing anti-inflammatory, not inventing anti-inflammatory modification of blood purification membrane, reducing the number of lymphocytes, etc., to reduce the formation of thrombus, increase hydrophilic performance, Safe and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
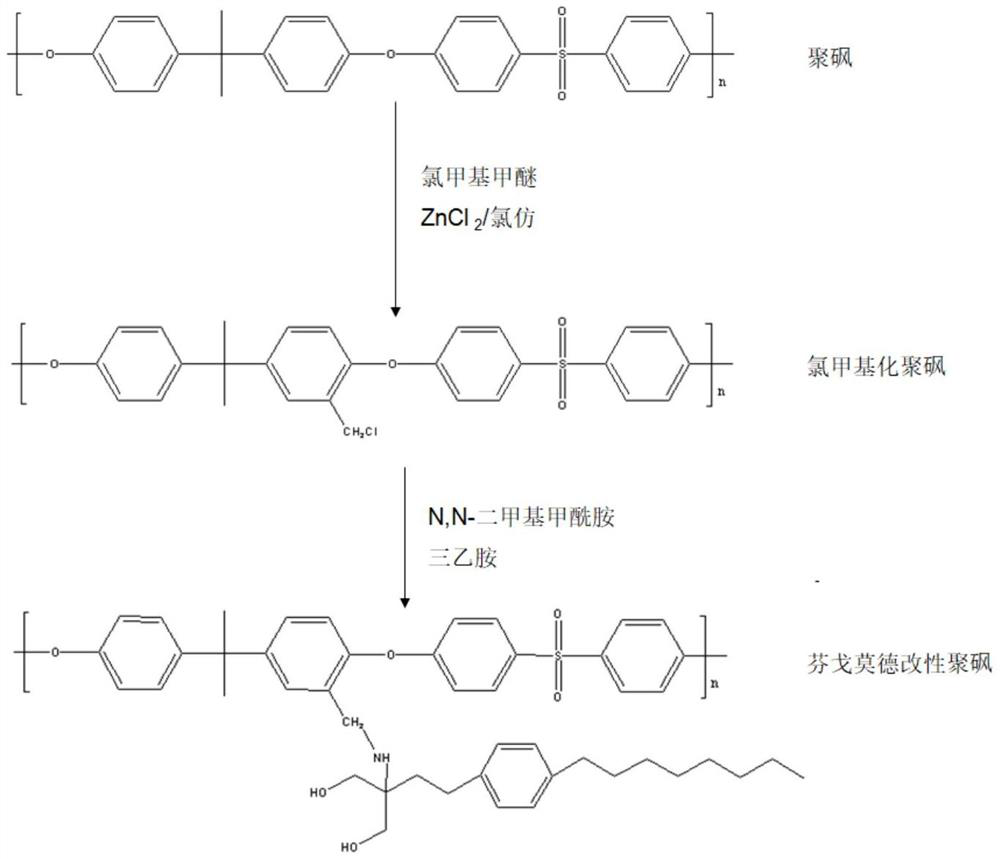
[0042] Specifically, on the one hand, an embodiment of the present invention provides a method for preparing a modified blood purification membrane, including the following steps:
[0043] (1) Preparation of chloromethylated polymer: Dissolve chloromethyl methyl ether, anhydrous zinc chloride and polymer in chloroform at a molar ratio of 10-15:2-5:1 and seal with nitrogen, then place Stirring and reacting in a water bath at 50-60°C for 2-8 hours to obtain the first reactant; filtering the first reactant, taking the filter residue, washing and drying to obtain the chloromethylated polymer;
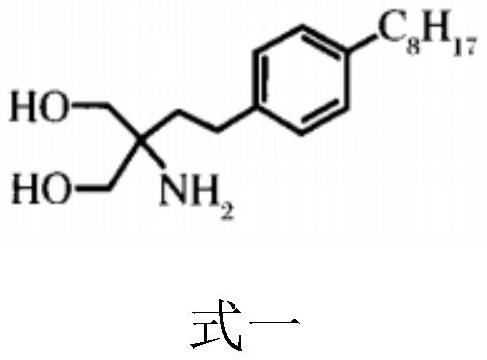
[0044] (2) Preparation of fingolimod modified polymer: dissolving fingolimod, triethylamine and the chloromethylated polymer of step (1) in N , in N-dimethylformamide, and then placed in a water bath at 50-60°C for 10-16 hours to obtain the second reactant; filter the second reactant, take the filter residue and wash it and dry it to obtain fingomole German modified polymer;
[0045](3) P...
Embodiment 1
[0066] A preparation method of a modified blood purification membrane, comprising the steps of:
[0067] (1) Preparation of chloromethylated polysulfone: chloromethyl methyl ether, anhydrous ZnCl 2 And polysulfone was dissolved in 20mL of chloroform with a molar ratio of 10:2:1 (the concentration of polysulfone was controlled within the range of 3%) and nitrogen blanketed, and then placed in a water bath at 50° C. for 2 h to obtain the first reactant; The first reactant was filtered, and the filter residue was taken, washed 3 times with absolute ethanol, and then dried in a vacuum oven at 60°C for 24 hours to obtain chloromethylated polysulfone;
[0068] (2) Preparation of fingolimod-modified polysulfone: dissolve fingolimod, triethylamine, and chloromethylated polysulfone in step (1) at a molar ratio of 8:1.5:1 in 15 mL of N, In N-dimethylformamide (the concentration of chloromethylated polysulfone is controlled in the range of 2%), and then placed in a 50°C water bath for 1...
Embodiment 2
[0072] A preparation method of a modified blood purification membrane, comprising the steps of:
[0073] (1) Preparation of chloromethylated polysulfone: chloromethyl methyl ether, anhydrous ZnCl 2 And polysulfone was dissolved in 20mL chloroform with a molar ratio of 15:5:1 (the concentration of polysulfone was controlled in the range of 6%) and nitrogen blanketed, and then placed in a water bath at 60° C. for 8 h to obtain the first reactant; The first reactant was filtered, and the filter residue was taken, washed 3 times with absolute ethanol, and then dried in a vacuum oven at 60°C for 24 hours to obtain chloromethylated polysulfone;
[0074] (2) Preparation of fingolimod-modified polysulfone: dissolve fingolimod, triethylamine, and chloromethylated polysulfone in step (1) at a molar ratio of 20:3:1 in 15 mL of N, N-dimethylformamide (the concentration of chloromethylated polysulfone is controlled in the range of 8%), and then placed in a 60°C water bath for 16h to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com