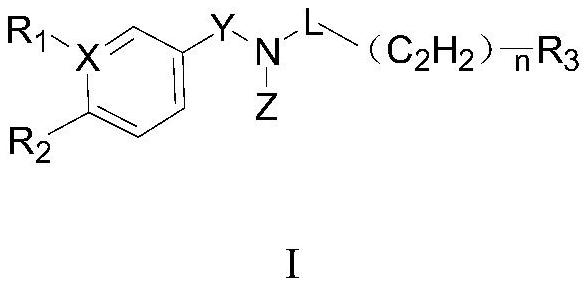
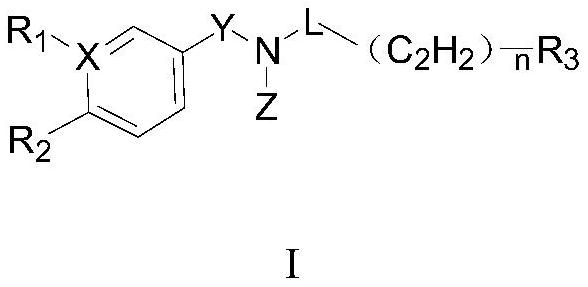
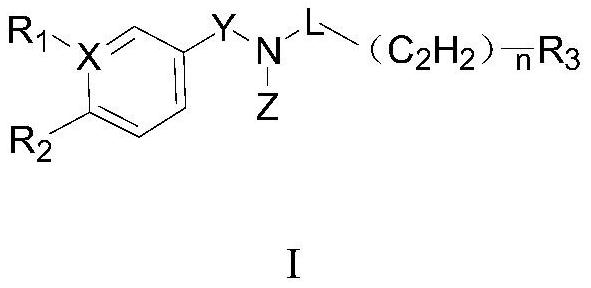
Aryl pentadiene amide aldehyde dehydrogenase inhibitor, and synthesis method and application thereof
An arylpentadienamide, aldehyde dehydrogenase technology, applied in the directions of anti-inflammatory agents, antitoxic agents, drug combinations, etc., can solve problems such as limited curative effect, achieve improved activity and water solubility, good druggability, and good development Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Preparation of N-(benzo[d][1,3]dioxol-5-ylmethyl)anthracene-9-carboxamide
[0090]
[0091] Anthracene-9-carboxylic acid (114 mg, 0.51 mmol) was dissolved in anhydrous DMF, HATU (235 mg, 0.6 mmol), 1 mL DIEA were added, and after stirring for 5 minutes, piperine (79 mg, 0.52 mmol) was added and reacted at room temperature for 24 h. TLC monitoring, after the reaction of the raw materials was completed, the reaction solution was poured into 50mL of 1N dilute hydrochloric acid solution, a large amount of white solids were precipitated, the mixed solution was allowed to stand overnight, filtered, and dried to obtain compound 1 with a yield of 84.1%.
Embodiment 2-4
[0093] Example 1 was repeated, with the difference that different raw materials were used to obtain compounds 2, 14, and 27 in Table 1. The details are as follows: Use 1-naphthoic acid to replace the raw material anthracene-9-carboxylic acid in Example 1 to obtain compound 2; use 2,5-dichlorobenzoic acid and 2,4-dichloronicotinic acid to replace the raw materials in Example 1 Anthracene-9-carboxylic acid and (6-methoxypyridin-3-yl)methanamine were used instead of the raw material piperine in Example 1 to prepare compounds 14 and 27, respectively.
Embodiment 5
[0095] Preparation of 2-((benzo[d][1,3]dioxol-5-ylmethyl)carbamoyl)-3-chlorobenzoic acid
[0096]
[0097] 1) Preparation of 2-chloro-6-((2-ethoxy-2-oxoethyl)carbamoyl)benzoic acid
[0098] 4-Chloroisobenzofuran-1,3-dione (274mg, 1.5mmol) was dissolved in 10mL ethyl acetate, stirred at room temperature to dissolve, and 2-aminoacetate ethyl ester (155mg, 1.5mmol) was added dropwise, Can produce a large amount of white solids immediately, TLC monitors, and after the reaction is completed, stand and filter, and dry to obtain the intermediate 2-chloro-6-((2-ethoxyl-2-oxoethyl)carbamoyl)benzoic acid , 90% yield.
[0099] 2) Preparation of methyl 2-(2-((benzo[d][1,3]dioxol-5-ylmethyl)carbamoyl)-3-chlorobenzamido)acetate
[0100] Dissolve the intermediate 2-chloro-6-((2-ethoxy-2-oxoethyl)carbamoyl)benzoic acid (213mg, 0.51mmol) in anhydrous DMF, add HATU (235mg, 0.6mmol ), 1mL DIEA, stirred for 5 minutes, added piperine (79mg, 0.52mmol), reacted at room temperature for 24h, mon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com