Active tumor targeting controlled-release drug nano-micelle and preparation method and application thereof
A tumor-targeting, nanomicelle technology, applied in the field of biological and medical nanomaterials, can solve the problems of poor tissue selectivity, limit the clinical application of photodynamic therapy, aggregation-induced quenching, etc., to overcome poor water solubility and increase retention time in vivo , increase the effect of accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
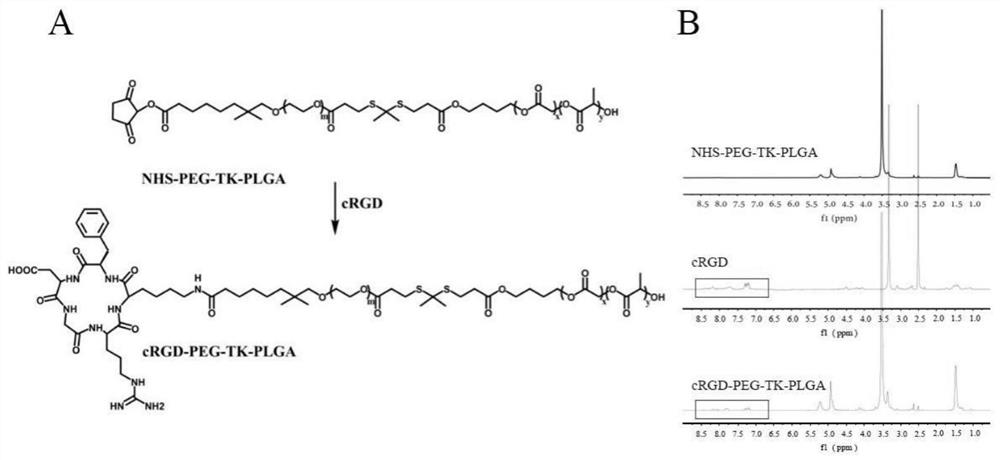
[0033] Embodiment 1. The present invention provides a nanomicelle with active tumor targeting and controlled release of drugs. The nanomicelle uses PEG-TK-PLGA as the basic skeleton and has cRGD modification on the surface.
[0034] Poly(lactic-co-glycolic acid) (PLGA) is the most commonly used biodegradable polymeric drug delivery vehicle today. In order to construct stimuli-responsive nanocarriers, the controlled-release drug nanosystems constructed with PLGA-based polymers have received a lot of attention. The thioketal bond (TK) can be specifically broken in response to the stimulation of reactive oxygen species, so as to achieve the localization of the tumor site and release chemotherapy drugs, effectively improving the utilization of anti-tumor drugs. In addition, polyethylene glycol (PEG) is a polymer material widely used for covalent modification of biopolymers such as proteins and peptides. PEG has the advantages of non-toxicity, non-immunogenicity, non-antigenicity,...
Embodiment 2
[0037] Example 2. Synthesis of nanomicelles that can actively target tumors in response to ROS stimulation
[0038] Concrete preparation method comprises the following steps:
[0039] (1) Synthesis of cRGD-PEG-TK-PLGA: The cRGD polypeptide is coupled with NHS-PEG-TK-PLGA through amidation reaction, and its synthetic route is as follows figure 1 As shown in A, 5 ml of NHS-PEG-TK-PLGA in dimethylformamide (DMF) was mixed into a DMF solution containing cRGD polypeptide (20 mg, 0.033 mmol). After reacting at room temperature for 24 hours, it was purified by ultrapure water dialysis (MWCO 3500) for 48 hours, and then freeze-dried to obtain cRGD-PEG-TK-PLGA. Coupling rate: 74.6%.
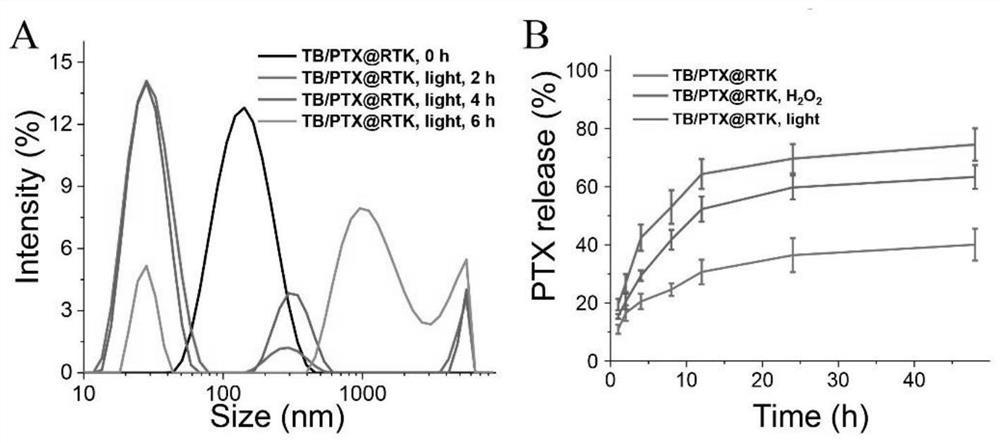
[0040] (2) Synthesis of ROS-responsive nanomicelles that can actively target tumors: PEG-TK-PLGA (9 mg) and cRGD-PEG-TK-PLGA (1 mg) were dissolved in THF / CHCl3 (v / v=1 :1, 1.5mL) solvent, and then slowly added ultrapure water (8.5mL). Then, it was dialyzed against ultrapure water for 1 d to prepare ROS...
Embodiment 3
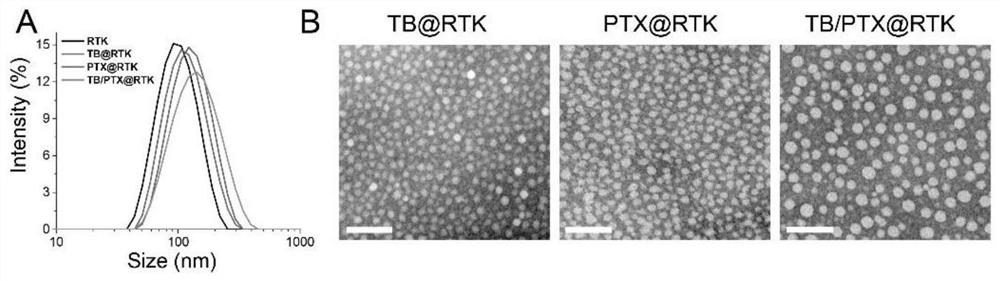
[0042] Example 3, Synthesis of nanomicelles loaded with AIE photosensitizer (TB) and (or) chemotherapeutic drug paclitaxel (PTX) capable of active tumor targeting
[0043] (1) Synthesis of TB@RTK nanomicelles: PEG-TK-PLGA (9 mg), cRGD-PEG-TK-PLGA (1 mg) and AIE photosensitizer (TB) (1 mg) were dissolved in THF / CHCl3 (volume ratio v / v=1:1, 1.5mL) solvent, dissolved in the dark, and then slowly added ultrapure water (8.5mL). Then, it was dialyzed against ultrapure water for 1 d to prepare nanomicelles (TB@RTK) loaded with AIE photosensitizer (TB) with active tumor targeting. The detection result of high performance liquid chromatography showed that its drug loading was 7.43%.
[0044] (2) Synthesis of PTX@RTK nanomicelles: PEG-TK-PLGA (9mg), cRGD-PEG-TK-PLGA (1mg) and PTX (1mg) were dissolved in THF / CHCl3 (v / v=1:1 , 1.5 mL) solvent, and then slowly added ultrapure water (8.5 mL). Then, it was dialyzed with ultrapure water for 1 d to prepare nanomicelles (PTX@RTK) loaded with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com