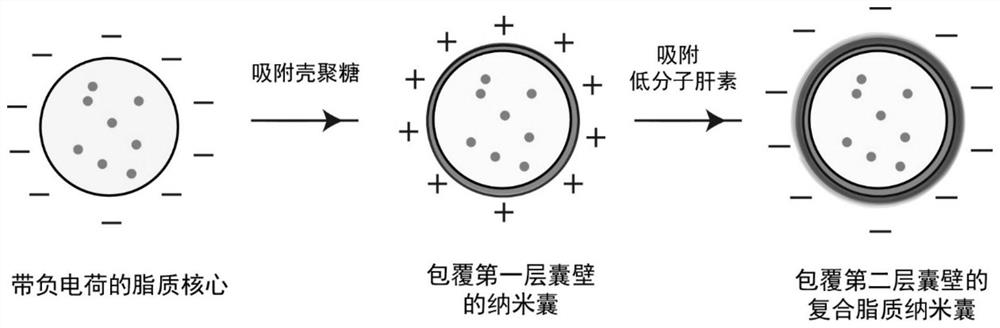
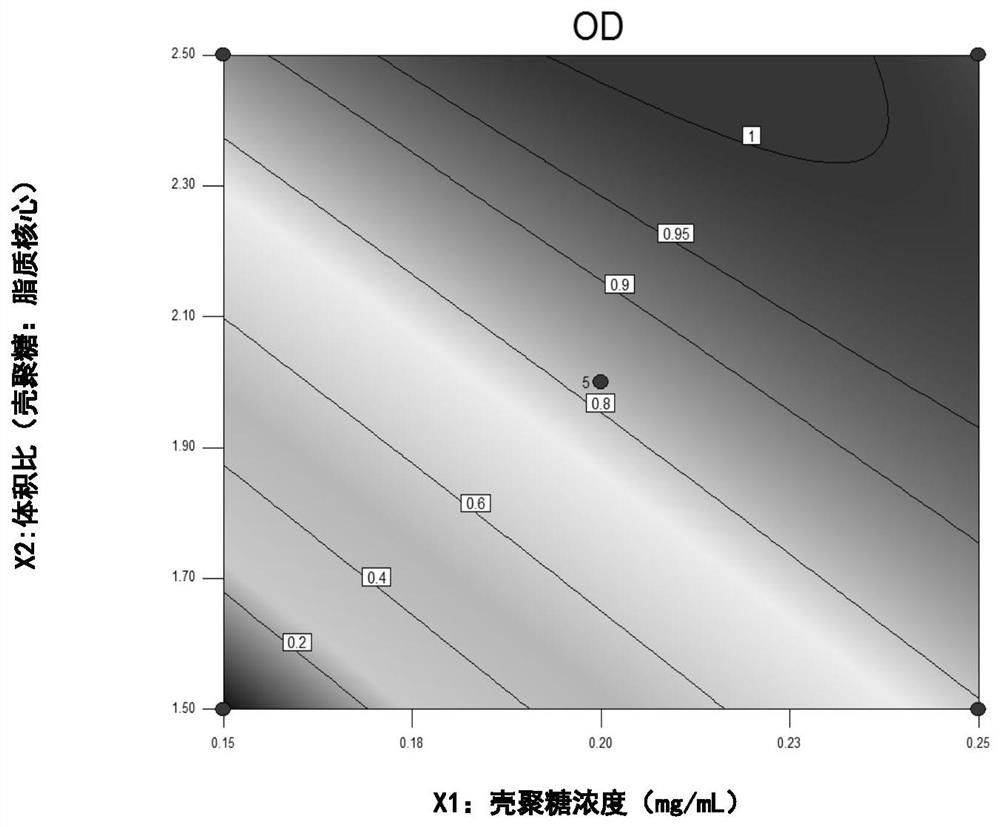
Composite lipid nanocapsule composition and preparation method and application thereof
A composite lipid and nanocapsule technology, which is applied in drug combination, capsule delivery, microcapsules, etc., can solve the problems of insufficient ability of targeted drug delivery, inapplicability of intravenous injection, and limitation of clinical application, etc., to improve drug stability Sex, improve solubility, improve the effect of treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
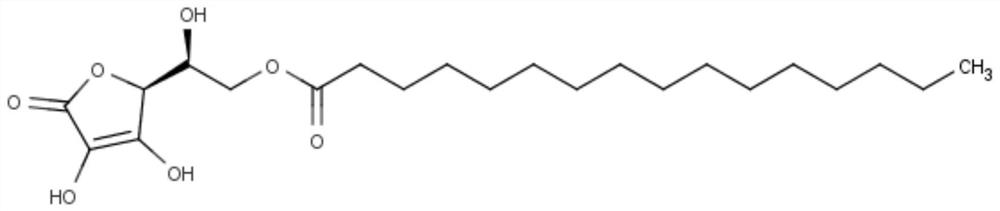
[0063] The regulation surface negative charge effect of embodiment 1 ascorbyl palmitate
[0064] Lipid cores containing different proportions of ascorbyl palmitate were prepared and compared with normal liposomes without ascorbyl palmitate.
[0065] Table 1 Formulation and characterization of different ascorbyl palmitate ratios
[0066]
[0067] 1. The preparation method of prescription A1 (ordinary liposome):
[0068] (1) Dissolve the prescribed amount of phospholipids (PC:80-85%) and cholesterol in chloroform-methanol (88:12) to obtain a clear and transparent solution, and remove the organic solvent by rotary evaporation to obtain a lipid film.
[0069] (2) Place the lipid film obtained in step 1 in a water bath at 40-60° C., slowly add 25 mL of phosphate buffer (pH=7) containing about 0.2% Tween 80 under stirring to obtain a thick emulsion. The coarse emulsion is homogenized under high pressure to obtain liposomes.
[0070] 2. Preparation method of prescriptions A2-A5...
Embodiment 2
[0076] Comparison of different phospholipids of embodiment 2
[0077] The effect of using phospholipids containing different proportions of phosphatidylcholine (PC: 80%-100%) on the preparation of lipid core was investigated.
[0078] Table 2 Formulation and characterization of different phospholipids
[0079]
[0080] Preparation:
[0081] (1) Dissolve the prescribed amount of phospholipids and ascorbyl palmitate in methanol to obtain a clear and transparent solution, remove the methanol by vacuum rotary evaporation in a water bath at 30-40°C, and collect the resulting precipitate, that is, the mixed membrane material.
[0082] (2) Dissolving the mixed film material and medium-chain oil obtained in step 1 in dichloromethane to obtain a uniform solution, and removing the dichloromethane by vacuum rotary evaporation in a water bath at 30-40° C. to obtain a lipid-film complex.
[0083] (3) Put the lipid membrane complex obtained in step 2 in a water bath at 40-60° C., slowly ...
Embodiment 3
[0085] Comparison of different oils and fats of embodiment 3
[0086] The effect of using different oils on the preparation of nanocapsule lipid core was investigated.
[0087] Table 3 Recipe and characterization of different oil phases
[0088]
[0089] Preparation:
[0090] (1) Dissolve the prescribed amount of phospholipids (PC≥98%) and ascorbyl palmitate in methanol to obtain a clear and transparent solution, remove the methanol by vacuum rotary evaporation in a water bath at 30-40°C, and collect the resulting precipitate, which is the mixed membrane material.
[0091] (2) Dissolving the mixed film material obtained in step 1 and the oil phase of the prescription amount in dichloromethane to obtain a uniform solution, and removing the dichloromethane by vacuum rotary evaporation in a 30-40°C water bath to obtain a lipid-film complex.
[0092] (3) Place the lipid membrane complex obtained in step 2 in a 40-60°C water bath, slowly add 50 mL of phosphate buffer (pH=7) co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com