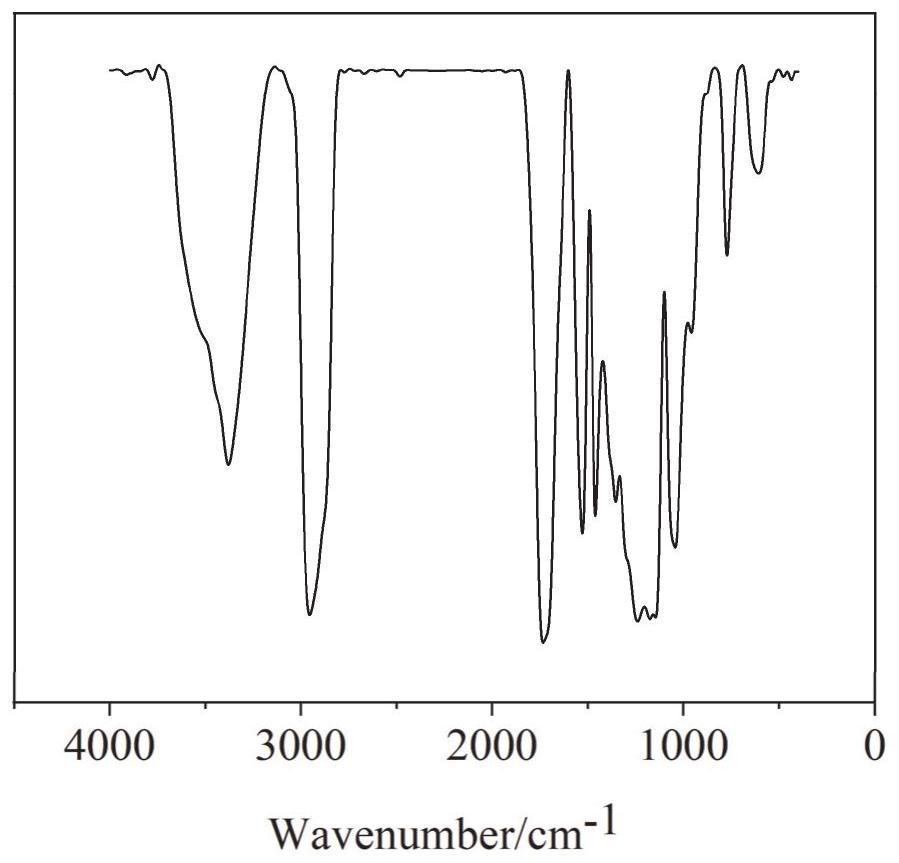
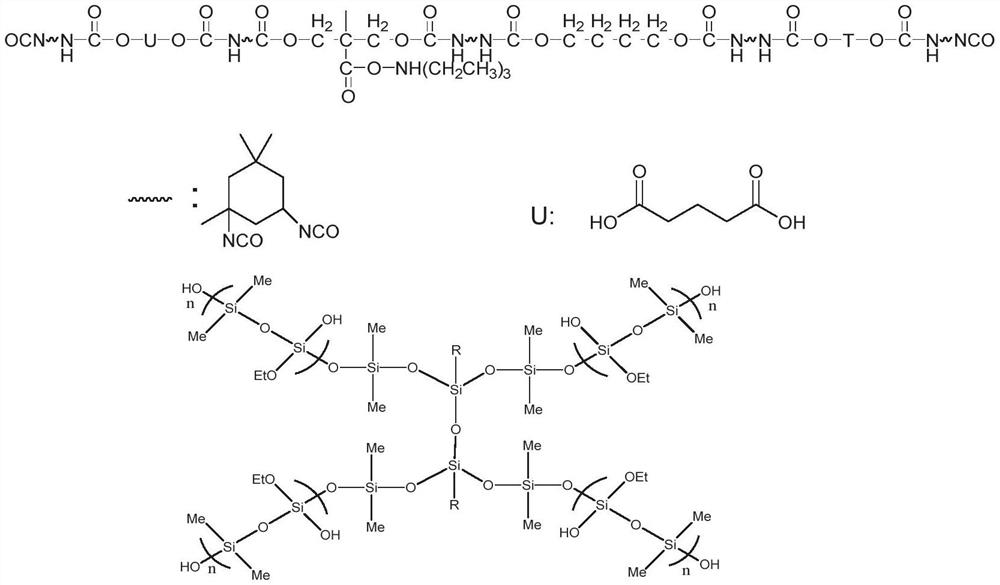
Hyperbranched organic amino silicon post-chain-extension modified waterborne polyurethane and preparation method thereof
A technology of water-based polyurethane and organic amino group, applied in the field of high-molecular water-based polyurethane, can solve the problems of insufficient mechanical properties change, unfavorable, limited degree of improvement, etc., and achieve good industrial production prospects, reduce production costs, and improve the effect of contact angle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In a four-neck flask equipped with a thermometer probe, serpentine condenser, mechanical stirring rod, and nitrogen inlet, add 15.0 g of isophorone diisocyanate (IPDI), 45.0 g of polyadipate-1,4-butanediol Ester diol (PBA, Mn=2000), 0.005g of dibutyltin dilaurate (DBTDL), need to pass nitrogen gas for 5min before adding the medicine. Ensure that the bottle is a nitrogen atmosphere, react at 80°C for 100min, then add 3.0g dimethylolpropionic acid (DMPA), react at 80°C for 90min, then add 1.0g trimethylolpropane (TMP), at 80°C Reaction at ℃ for 60 minutes, when the -NCO of the system measured by the n-dibutylamine method reaches the theoretical requirement, lower the temperature of the system to 45 ℃, add 2.04g of triethylamine, and react at 40 ℃ for 20 minutes to neutralize the acid in the system , A total of 30ml of organic solvent acetone was added during the reaction; 1.0g of diethylenetriamine was added to 120ml of deionized water, emulsified at 30°C for 10min to obt...
Embodiment 2
[0054] In a four-neck flask equipped with a thermometer probe, serpentine condenser, mechanical stirring rod, and nitrogen inlet, add 15.0 g of isophorone diisocyanate (IPDI), 40.0 g of polyadipate-1,4-butanediol Ester diol (PBA, Mn=2000), 0.005g of dibutyltin dilaurate (DBTDL), need to pass nitrogen gas for 5min before adding the medicine. Ensure that the bottle is a nitrogen atmosphere, react at 80°C for 100min, then add 3.2g dimethylolpropionic acid (DMPA), react at 80°C for 90min, then add 1.2g trimethylolpropane (TMP), at 80°C Reaction at ℃ for 60 minutes, when the -NCO of the system measured by the n-dibutylamine method reaches the theoretical requirement, lower the temperature of the system to 45 ℃, add 2.18g of triethylamine, and react at 40 ℃ for 20 minutes to neutralize the acid in the system , A total of 25ml of organic solvent acetone was added during the reaction; 0.6g of diethylenetriamine was added to 120ml of deionized water, and emulsified at 30°C for 10min to...
Embodiment 3
[0056] In a four-necked flask equipped with a thermometer probe, serpentine condenser, mechanical stirring rod, and nitrogen inlet, add 20.0 g of isophorone diisocyanate (IPDI), 60.0 g of polyadipate-1,4-butanediol Ester diol (PBA, Mn=2000), 0.005g of dibutyltin dilaurate (DBTDL), need to pass nitrogen gas for 5min before adding the medicine. Ensure that the bottle is a nitrogen atmosphere, react at 80°C for 100min, then add 4.0g of dimethylolpropionic acid (DMPA), react at 80°C for 90min, then add 1.5g of trimethylolpropane (TMP), at 80°C Reaction at ℃ for 60 minutes, when the -NCO of the system measured by the n-dibutylamine method reaches the theoretical requirement, lower the temperature of the system to 45 ℃, add 2.35g of triethylamine, and react at 40 ℃ for 20 minutes to neutralize the acid in the system , A total of 35ml of organic solvent acetone was added during the reaction; 1.8g of diethylenetriamine was added to 120ml of deionized water, and emulsified at 30°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile stress | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com