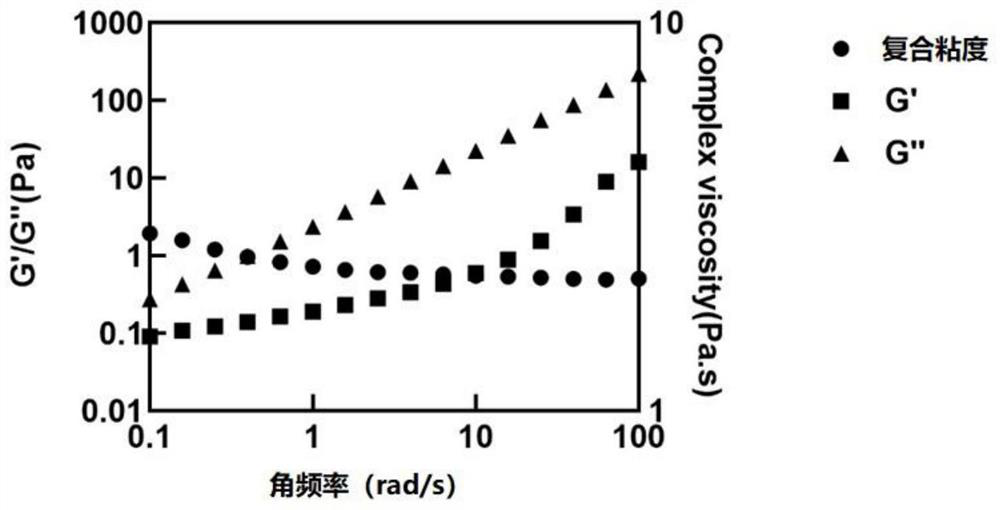
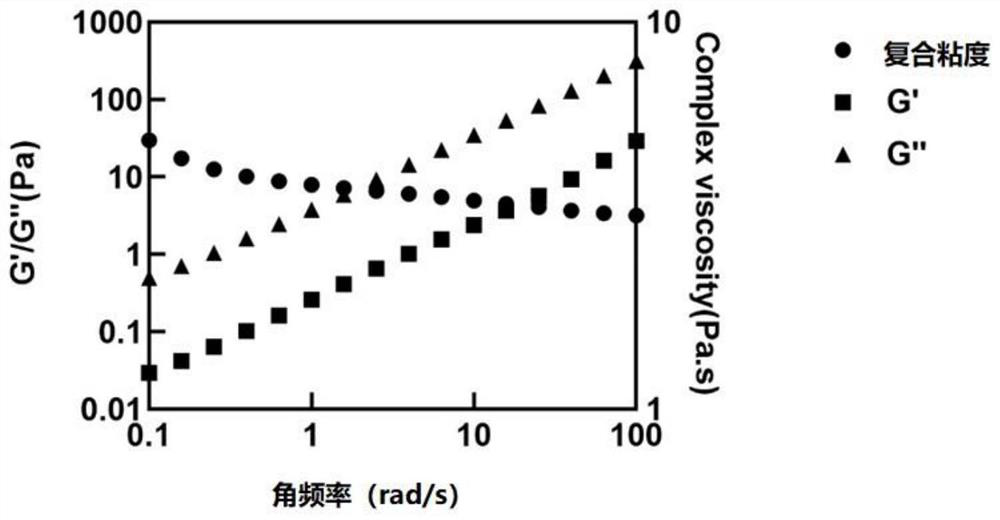
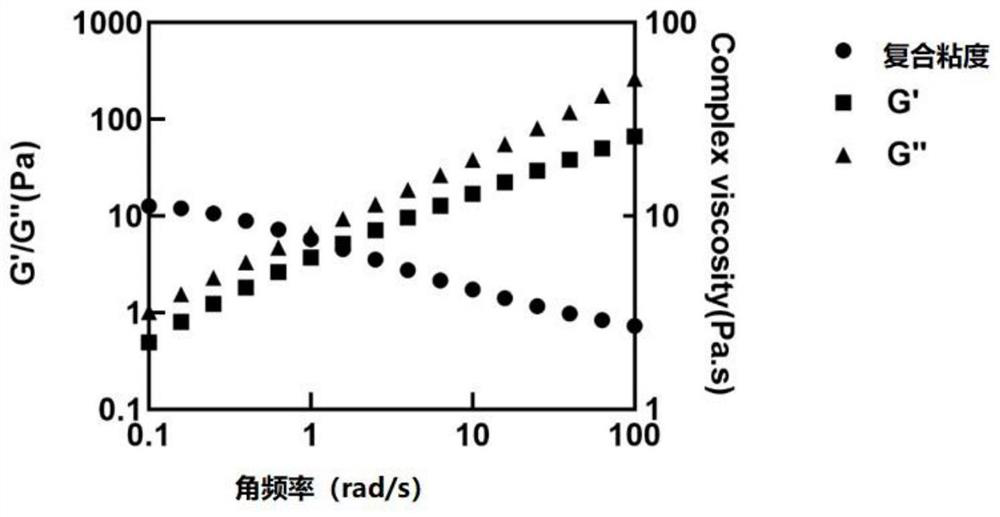
Small molecule drug sustained-release delivery system
A drug and sustained-release carrier technology, which is applied in the field of small-molecule drug sustained-release drug delivery system, can solve the problems of unsatisfactory incision drug administration, difficult-to-degrade sustained-release carrier, complicated preparation process, etc., and achieve good drug safety and tolerance Sexuality, improved sustained-release effect, good patient tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Different types of alcohol on the viscosity of the composition
[0083] The composition is formulated according to Table 1-1, in a heated state in the SPC was dissolved BA, were added to glycerol, propylene glycol, PEG200, PEG400, PEG600, heated and stirred until a homogeneous solution was allowed to stand at room temperature to investigate different types of alcohol on the viscosity of the system Influence. Using a rotor No. 14, 50rpm rotational speed detecting viscosity of the composition, the results shown in Table 1-2.
[0084] Table 1-1 compositions containing the active ingredient
[0085]
[0086] Table 1-2 in solution viscosity of the composition and
[0087]
[0088] The inventors have surprisingly found that the addition of glycerol phospholipid solution, can significantly increase the viscosity of the composition, propylene glycol and polyethylene glycol of different molecular weight and viscosity of the composition not significantly increase.
Embodiment 2
[0090] Glycerol / organic solvent / slow release carrier ratio of viscosity of the composition
[0091] Table 2-1 and 2-2 was formulated compositions, in a heated state and the SPC was dissolved in the proportion of different types of solvents, glycerin, heated and stirred until a homogeneous solution was allowed to stand at room temperature, the study group of the compound materials the viscosity ratio Impact.
[0092] TABLE 2-1 Viscosity detection result of the composition containing different solvents
[0093]
[0094]
[0095] TABLE 2-2 Viscosity detection result of the composition containing different solvents
[0096]
Embodiment 3
[0098] Study of composition homogeneity
[0099] Different viscosities taken in Tables 2-1 and 2-2 in compositions PE tube, centrifuged at 9000rpm 15min, the solution state was observed after centrifugation. The results are shown in Table 3-1 and Table 3-2.
[0100] Table 3-1 different solvents (benzyl alcohol) composition uniformity of results
[0101]
[0102] Table 3-2 different solvents (ethanol) results uniformity of composition
[0103]
[0104] From the results, the samples were centrifuged at high speed in the state of different viscosities no delamination, was homogeneous state, indicating good physical stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com