Toll-like receptor stimulant requinimod derivative and preparation and application thereof
A receptor agonist, Requinimod technology, applied in the field of medical materials, can solve the problems of low bioavailability, difficult to achieve ideal efficacy, limited application, etc., achieve huge application potential, and improve adverse pharmacokinetics. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of R848-HSEA
[0056]R848-HSEA is prepared by reacting R848 amino group with triphosgene-activated HSEA.
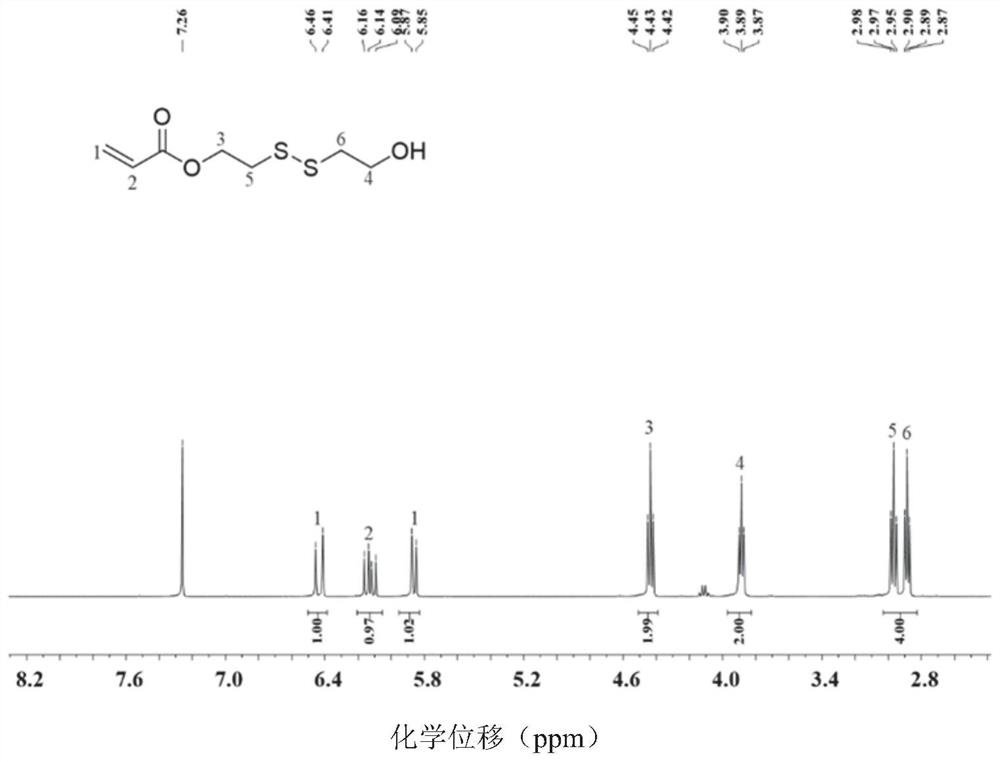
[0057] Preparation of HSEA:
[0058] Weigh 0.065mmol of hydroquinone, 20mmol of triethylamine, and 13mmol of 2-hydroxyethyl disulfide into a reaction vessel, add 200mL of anhydrous THF, and stir in an ice-water bath; extract 100mL of anhydrous THF with a glass needle into In the constant pressure dropping funnel, weigh 13mmol of acryloyl chloride and add it to the constant pressure dropping funnel; in the ice-water bath, slowly add acryloyl chloride dropwise for about 1 hour, and then transfer to room temperature for reaction; react for 24 hours Afterwards, stop the reaction, filter to remove the generated salt, spin the solvent to dryness, add dichloromethane, wash with saturated brine three times, then wash with pure water once, after the organic phase is dried with anhydrous magnesium sulfate, concentrate, and use silica gel column pur...
Embodiment 2
[0062] Embodiment 2: the preparation of R848-HSEMA:
[0063] R848-HSEA is prepared by reacting R848 amino group with triphosgene-activated HSMEA.
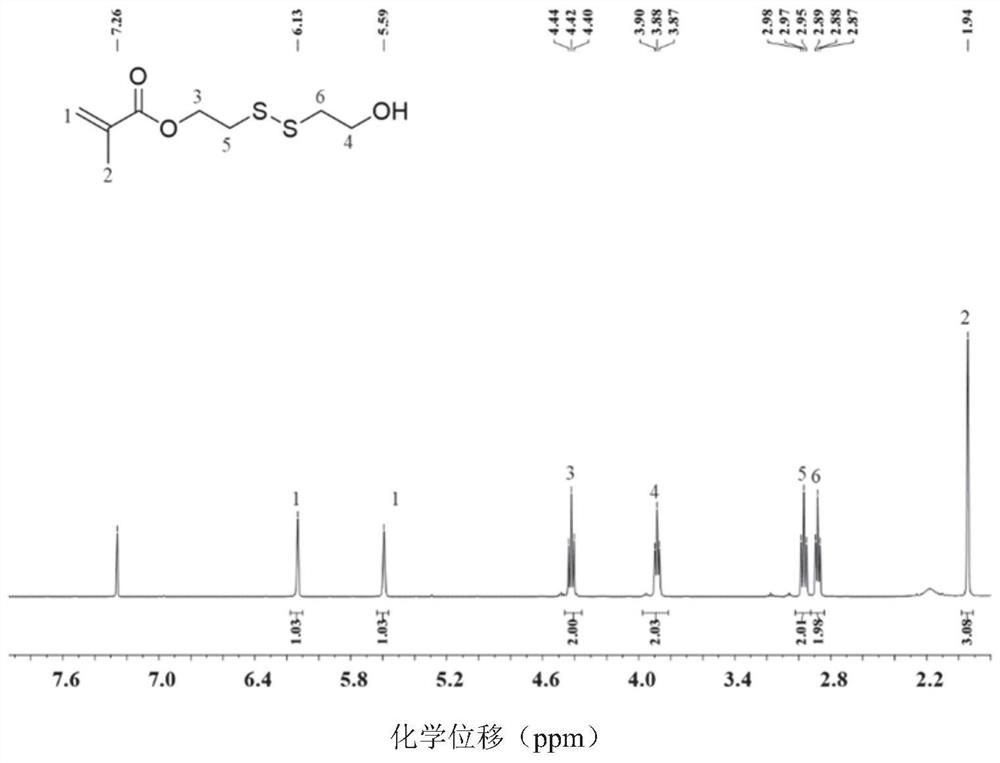
[0064] Preparation of HSEMA:
[0065] Weigh 0.065mmol of hydroquinone, 20mmol of triethylamine, and 13mmol of 2-hydroxyethyl disulfide into a reaction vessel, add 200mL of anhydrous THF, and stir in an ice-water bath; extract 100mL of anhydrous THF with a glass needle into In the constant pressure dropping funnel, weigh 13mmol methacryloyl chloride and add it to the constant pressure dropping funnel; in the ice-water bath, slowly add methacryloyl chloride dropwise for about 1 hour, and transfer to room temperature after the dropwise addition reaction; stop the reaction after 24 hours of reaction, filter to remove the generated salt, spin the solvent to dryness, add dichloromethane, wash three times with saturated saline, and then wash once with pure water, dry the organic phase with anhydrous magnesium sulfate, concentrate, and us...
Embodiment 3
[0069] Embodiment 3: Preparation of R848-TEGA
[0070] R848-TEGA is prepared by reacting R848 amino group with triphosgene-activated TEGA.
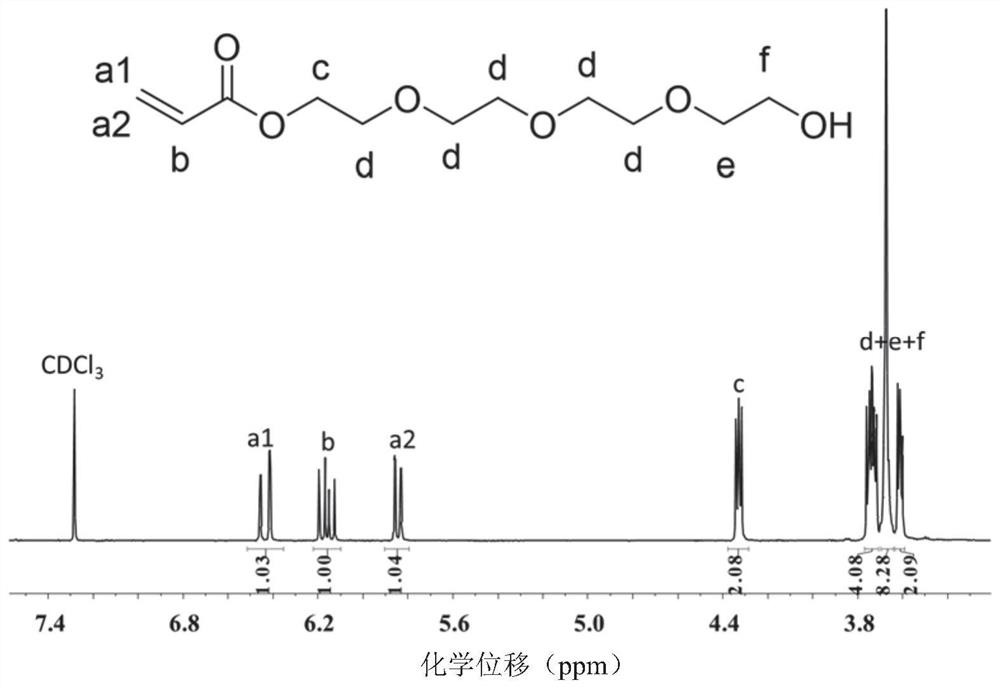
[0071] Preparation of TEGA:
[0072] Weigh 0.065mmol of hydroquinone, 20mmol of triethylamine, and 13mmol of tetraethylene glycol into a reaction vessel, add 200mL of anhydrous THF, and stir in an ice-water bath; extract 100mL of anhydrous THF with a glass needle at a constant pressure In the dropping funnel, weigh 13mmol of acryloyl chloride and add it to the constant pressure dropping funnel; in the ice-water bath, slowly add acryloyl chloride dropwise for about 1 hour. After the dropwise addition, transfer to room temperature for reaction; stop after 24 hours reaction, filtered to remove the generated salt, the solvent was spin-dried, added dichloromethane, washed three times with saturated brine, and then washed once with pure water, and the organic phase was dried with anhydrous magnesium sulfate, concentrated, and purified using a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com