Process for preparing bis(fluorosulfonyl) imide
A technology of fluorosulfonyl and imide, applied in the field of bisimide preparation, which can solve the problem of increasing carbon footprint
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
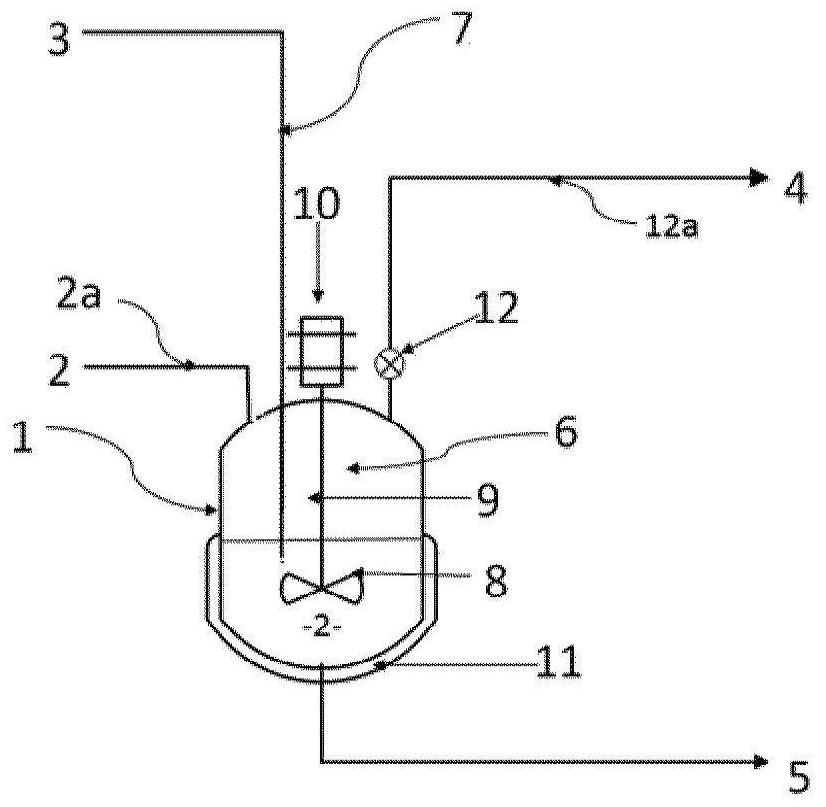
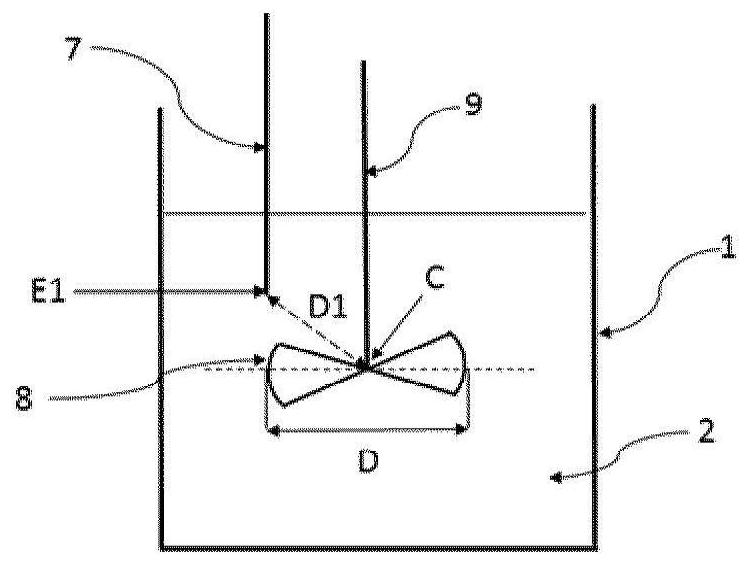
[0032] According to a particular embodiment, said liquid phase A2 comprises bis(halosulfonyl)imide but is free of organic solvents. Thus, step ii) of fluorination of the bis(halosulfonyl)imide to bis(fluorosulfonyl)imide is carried out in the absence of an organic solvent.
[0033] According to an alternative particular embodiment, said liquid phase A2 comprises a bis(halosulfonyl)imide and an organic solvent. The organic solvent SO1 may be selected from esters, nitriles, ethers, aromatic solvents, carbonates, cyclic or heterocyclic solvents and mixtures thereof. Preferably, the organic solvent SO1 is selected from methyl acetate, butyl acetate, ethyl acetate, propyl acetate, isopropyl acetate, butyronitrile, valeronitrile, benzonitrile, diisopropyl ether, 2-methyl acetate Oxy-2-methylbutane, cyclopentyl methyl ether, benzene, toluene, chlorobenzene, dichlorobenzene, xylene, ethylbenzene, 1,4-dioxane, dimethyl carbonate, ethylene Ethyl carbonate, sulfolane and mixtures there...
Embodiment 1
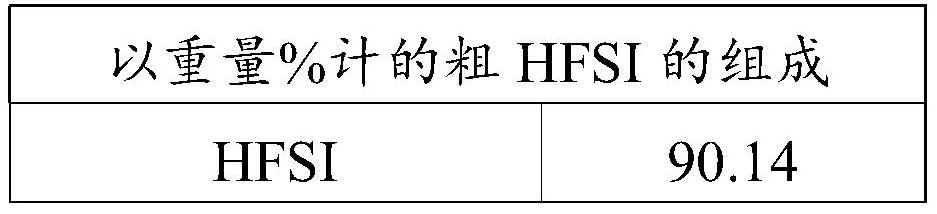
[0095]Into a stirred 1 liter reactor were introduced 394 g of liquid bis(chlorosulfonyl)imide (HCSI) and 19.7 g of liquid 1,4-dioxane. The weight ratio between 1,4-dioxane and HCSI was 5%. The mixture was agitated using a turbine with 6 pitched blades and allowed to reach 40 °C before introducing hydrofluoric acid. The reaction is carried out by adjusting the temperature of the reaction medium at 40° C. and by continuous injection of gaseous HF. Gaseous HF is injected slowly directly into the liquid reaction medium through a dip tube. The total amount of HF injected was 110 g, which corresponds to a molar ratio of HF to HCSI of 3. The rate of introduction of gaseous HF was adjusted at 37 g / h. The reaction time was 3 hours. The reaction is accompanied by the formation of HCl, which is continuously removed from the reactor. The gas leaving the reactor is sent to a water trap. When all the HF has been introduced, a nitrogen stream with a flow rate of 50 l / h is introduced in...
Embodiment 2
[0100] Into a stirred 1 liter reactor were introduced 397 g of liquid bis(chlorosulfonyl)imide (HCSI) and 12 g of liquid 1,4-dioxane. The weight ratio between 1,4-dioxane and HCSI was 3%. The mixture was agitated using a turbine with 6 pitched blades and allowed to reach 45°C before introducing hydrofluoric acid. The reaction is carried out by adjusting the temperature of the reaction medium at 45° C. and by continuous injection of gaseous HF. Gaseous HF is injected slowly directly into the liquid reaction medium through a dip tube. The total amount of HF introduced was 100 g, which corresponds to a molar ratio of HF to HCSI of 2.7. The introduction rate of gaseous HF was adjusted at 38 g / h. The reaction time was 2 hours and 40 minutes. The reaction is accompanied by the formation of HCl, which is continuously removed from the reactor. The gas leaving the reactor is sent to a water trap. When all the HF has been introduced, a nitrogen stream with a flow rate of 50 l / h is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com