Preparation method of gemifloxacin side chain compound
A technology of gemifloxacin and compounds, applied in the field of preparation of gemifloxacin side chain compounds, can solve the problems of unfavorable industrial production, unfavorable industrial production, long route, etc., and achieve the effect of low cost, easy realization and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the preparation of gemifloxacin side chain
[0059] (1) Synthesis of Compound V
[0060] Purchasing compound IV, take 105.0g compound IV, 50.1g (1.2eq) methoxyamine hydrochloride and 1000ml methanol into a 2L reaction flask, then add 47.5g (1.2eq) pyridine as catalyst, at 20~25°C React for 5h, TLC monitors the reaction, after the reaction is completed, distill to dryness under reduced pressure, add water and dichloromethane to separate layers, wash the organic phase with saturated sodium bicarbonate solution and saturated brine successively, dry over anhydrous sodium sulfate, and distill to dryness under reduced pressure , to obtain compound V (117.8 g, yield 98.4%).
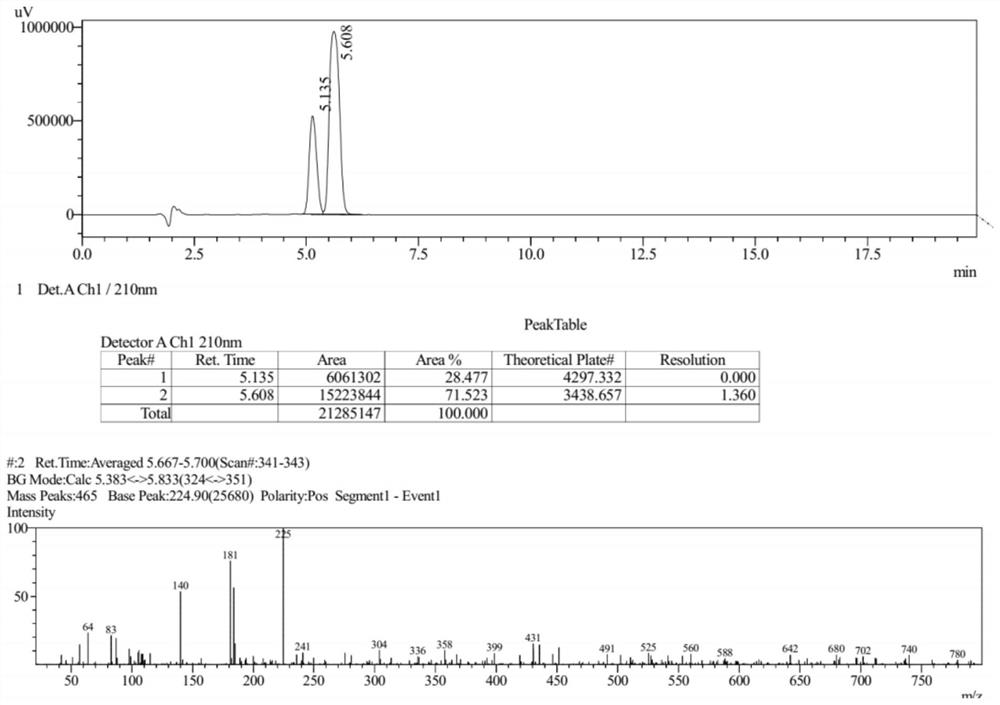
[0061] The LC-Ms spectrum of compound V is as follows figure 1 shown.
[0062] (2) Synthesis of compound VI
[0063] Add 20g of compound V, 200ml of methanol and 3g of 10% palladium carbon into a stainless steel hydrogenation kettle, and react for 2h at 20-25°C under a hydrogen atmosphere ...
Embodiment 2
[0090] Embodiment 2, the preparation of gemifloxacin
[0091] Add 14.1 mg (5 mmol) of 1-cyclopropyl-7-chloro-6-fluoro-4-oxo-1,4-dihydro[1,8]naphthyridine-3-carboxylic acid and 15 ml of water into the reaction flask , add triethylamine, then dropwise add 10.8g (5mmol) of 4-aminomethyl-3-methoxyiminopyrrole prepared in Example 1, react at 20-25°C for 15 hours, filter, separate the precipitated solid and Drying gave 16.7 g (yield: 85%) of gemifloxacin (compound (III)).
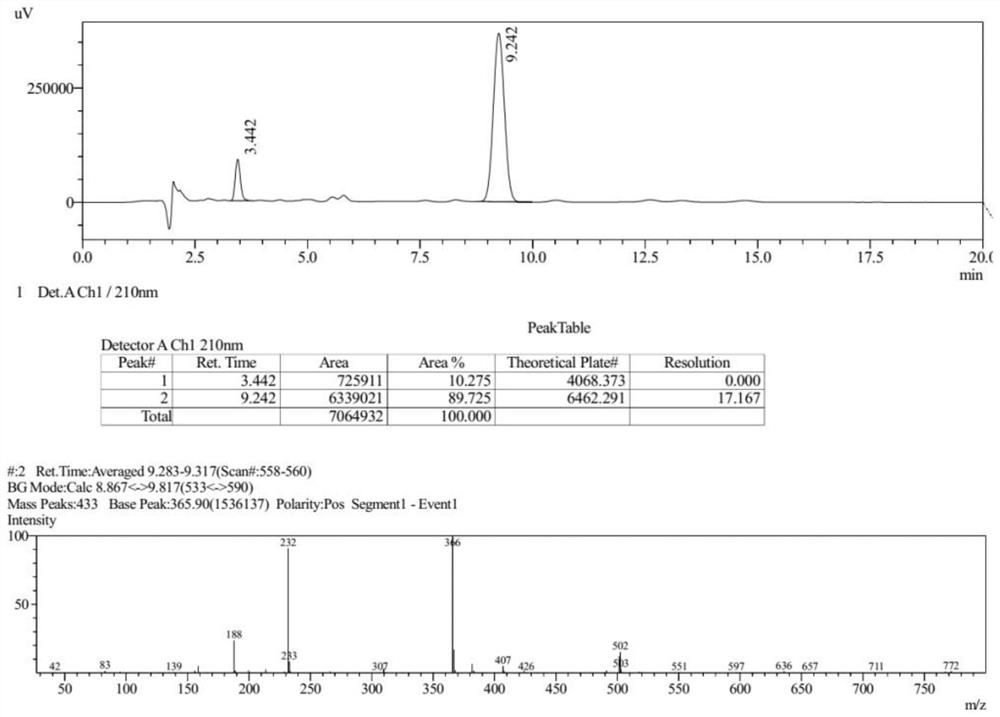
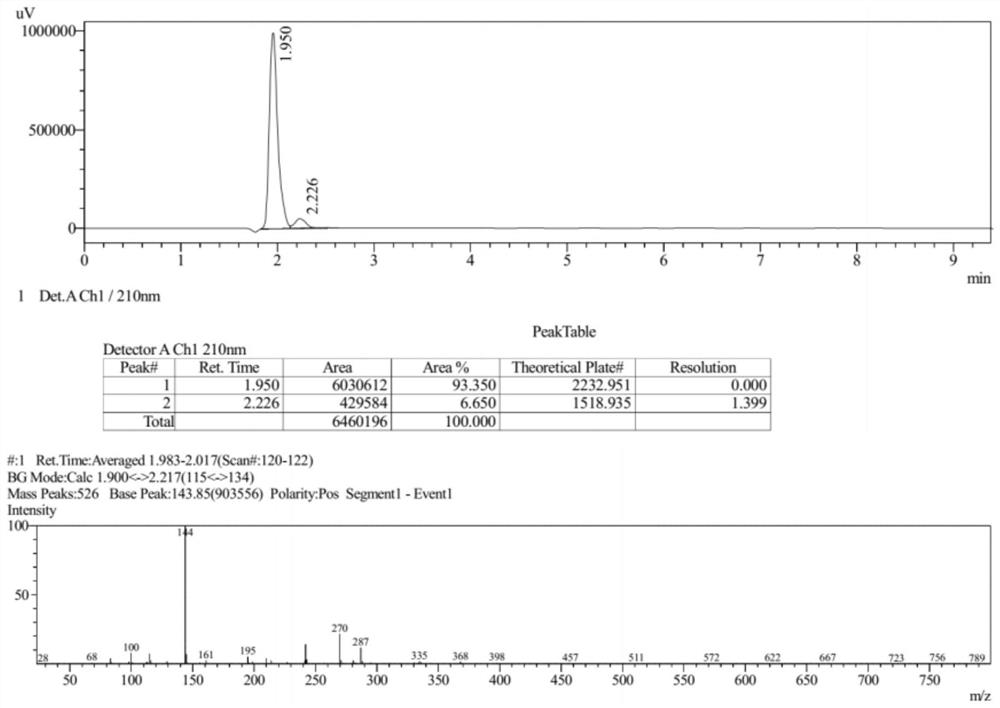
[0092] The Ms collection of collection of collections, NMR collection of collection of collections and HPLC collection of collections of the compound (III) prepared in the present embodiment are respectively as follows Figure 6-Figure 8 shown.
[0093] Wherein, the detection condition of HPLC is as follows:
[0094] Chromatographic column: CAPCELL PAK C18 150×4.6mm×5um;
[0095] Injection volume: 20ul;
[0096] Temperature: 45°C;
[0097] Wavelength: 270nm;
[0098] Flow rate: 1.2ml / min;
[0099] Mobile ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com