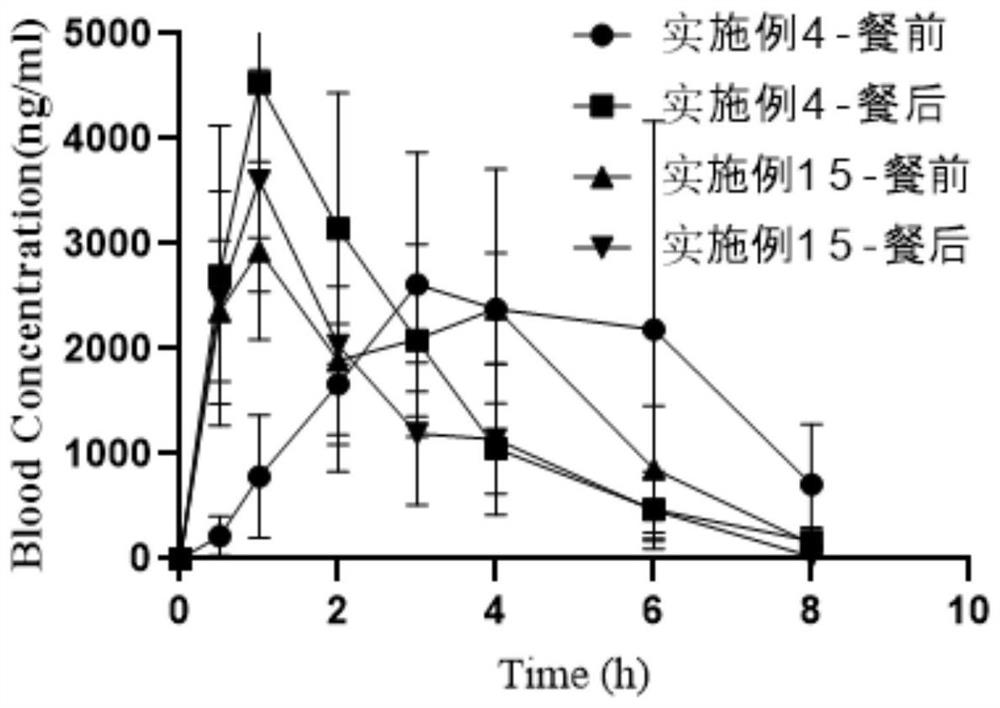
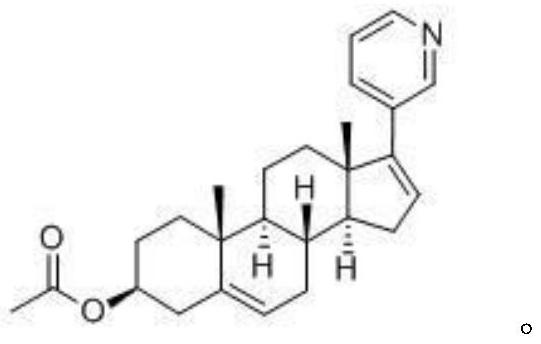
Oral abiraterone pharmaceutical composition and preparation method and use thereof
A technology of abiraterone and abiraterone acetate, applied in the field of oral abiraterone pharmaceutical composition and its preparation, can solve the problems of product stability and curative effect, insufficient bioavailability, inconvenient administration, etc. , to achieve the effects of improving bioavailability and drug loading, realizing large-scale industrial production, and eliminating differences in blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] This embodiment provides a pharmaceutical composition containing abiraterone acetate, and the proportion of each component in the combination is calculated by mass fraction, including the following components:
[0164] Name Proportion (mass ratio)
[0165] Abiraterone Acetate 28.6%
[0166] Oleic acid 71.4%
[0167] Preparation method: Add abiraterone acetate to oleic acid, stir or ultrasonically dissolve it completely to form a uniform and clear solution.
[0168] A large amount of bile salt is also secreted in the gastrointestinal tract of the human body, and the phospholipids in the human body can self-emulsify oil-soluble substances. This embodiment is the most simplified combination of the embodiments of the present invention.
Embodiment 2
[0170] This embodiment provides a pharmaceutical composition containing abiraterone acetate, and the proportion of each component in the combination is calculated by mass fraction, including the following components:
[0171]
[0172] Preparation method: Mix oleic acid, polyoxyethylene 40 hydrogenated castor oil, and propylene glycol evenly, then add abiraterone acetate, stir or ultrasonically dissolve it completely, and form a uniform and clear solution.
Embodiment 3
[0174] This embodiment provides a pharmaceutical composition containing abiraterone acetate, and the proportion of each component in the combination is calculated by mass fraction, including the following components:
[0175]
[0176] Preparation method: Mix oleic acid, polyoxyethylene 35 castor oil, and propylene glycol evenly, then add abiraterone acetate, stir or ultrasonically dissolve it completely, and form a uniform and clear solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com