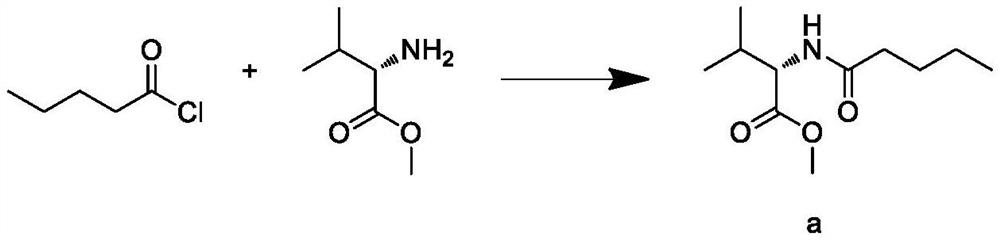
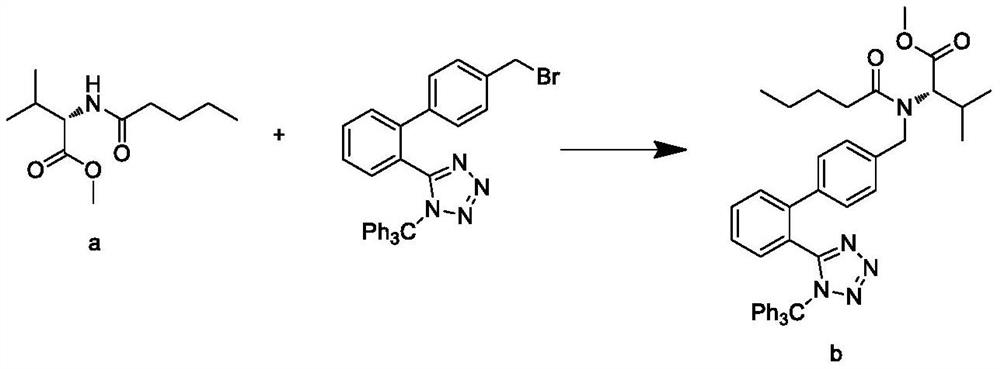
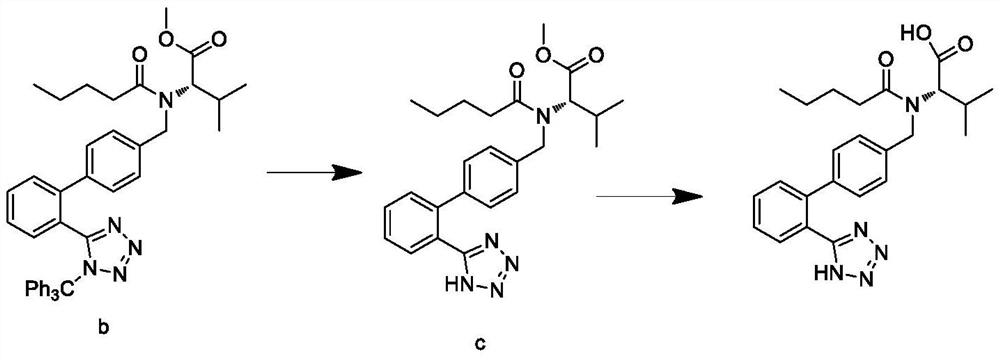
Synthesis process of valsartan
A synthesis process and valsartan technology are applied in the field of valsartan synthesis technology, can solve the problems of high pollution and high risk, and achieve the effects of improving product purity, improving production efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The chiral fixation is made by the following steps:
[0029] Step S1, 1.0 g Quinin is dispersed in 60 ml of toluene, reflux reaction for 3 h, after the reaction is completed, cooled to room temperature, add 0.63 g of 4-nitrophenyl chloroformate, at room temperature, rotation speed 60R / min stirring reaction 24h After the reaction, filtered, filter cake was washed with toluene, n-hexane was washed, and then dried in vacuo to constant weight at 40 ° C to give intermediate 1;
[0030]Step S2, 0.63 g of intermediate 1, 0.3 g of iron powder and 68 ml of anhydrous ethanol were added to the reactor, and after refluxing for 3 h, a hydrochloric acid solution was added, and the reaction liquid pH was 7, continued to react 1 h, and the reaction was completed, filtered After extraction of ethyl acetate, evaporation, to give intermediate 2, hydrochloric acid solution mass fraction of 17%;
[0031] Step S3, 5.8 g of intermediate 2, 1.1 g of chloroacetrazine and 68.7 ml of tetrahydrofura...
Embodiment 2
[0035] The chiral fixation is made by the following steps:
[0036] Step S1, 1.0 g Quinnin is dispersed in 62 ml of toluene, reflux reaction for 3 h, after the reaction is completed, cooled to room temperature, add 0.64 g of 4-nitrophenyl chloroformate, at room temperature, rotational speed 70R / min stir stock 24 h After the reaction, filtered, filter cake was washed with toluene, n-hexane was washed, and then dried in vacuo to constant weight at 40 ° C to give intermediate 1;
[0037] Step S2, 0.64 g of intermediate 1, 0.3 g of iron powder and 70 ml of anhydrous ethanol were added to the reactor, and after refluxing for 4 h, a hydrochloric acid solution was added, and the reaction liquid pH was 7, continued to react 1 h, and the reaction was completed, filtered After extraction of ethyl acetate, evaporation, to give intermediate 2, hydrochloric acid solution mass fraction of 17%;
[0038] Step S3, 6.1 g of intermediate 2, 1.1.2 g of chloroacetamic chloride and 72.3 ml of tetrahy...
Embodiment 3
[0042] The chiral fixation is made by the following steps:
[0043] Step S1, 1.0 g Quinnon is dispersed in 65 ml of toluene, reflux reaction for 3 h, after the reaction, cool to room temperature, add 0.65 g of 4-nitrophenyl chloroformate, at room temperature, rotation speed 80R / min stirring reaction 24h After the reaction, filtered, filter cake was washed with toluene, n-hexane was washed, and then dried in vacuo to constant weight at 40 ° C to give intermediate 1;
[0044] In step S2, 0.65 g of intermediate 1, 0.3 g of iron powder and 74 ml of anhydrous ethanol were added to the reactor, and after refluxing reaction for 5 h, a hydrochloric acid solution was added, and the reaction liquid pH was 8, and the reaction was continued for 1 h, and the reaction was completed, filtered After extraction of ethyl acetate, evaporation, to give intermediate 2, hydrochloric acid solution mass fraction of 17%;
[0045] Step S3, 6.3 g of intermediate 2, 1.3 g of chloroacetyl chloride and 74.1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com