Synthesis method of 2-trifluoromethyl benzamide
A technology for trifluoromethylbenzamide and trifluoromethylbenzene cyanide, which is applied in the field of organic chemical synthesis, can solve the problems of low 2-trifluoromethylbenzamide yield, large pollution and the like, and achieves easy recovery, The effect of less three wastes and good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
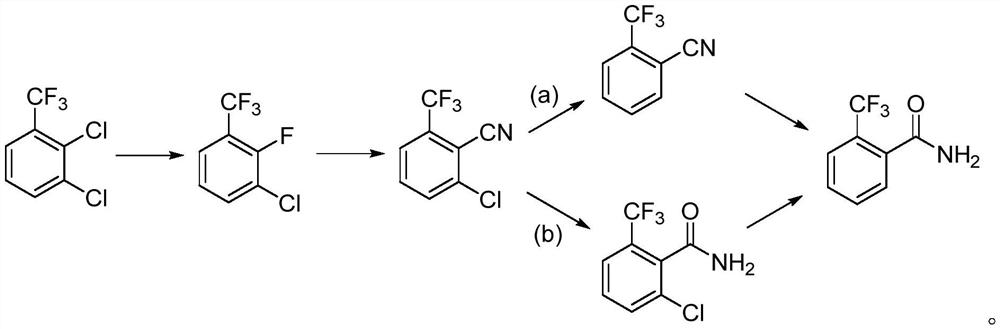
[0036] 1) Preparation of 2-fluoro-3-chlorobenzotrifluoride
[0037] Add 215g of 2,3-dichlorobenzotrifluoride, 73g of potassium fluoride, 7g of triphenylphosphine bromide and 430mL of 1,3-dimethylimidazolidinone into a 1000mL three-necked flask equipped with a rectification column, and stir to raise the temperature Reflux reaction. After 1 hour of reaction, 185 g of a colorless transparent liquid began to evaporate slowly, and the content of GC analysis was 93.9%, which was 2-fluoro-3-chlorobenzotrifluoride. The yield of crude product is 93.2%. The crude product was directly used in the next reaction without purification.
[0038] 2) Preparation of 2-chloro-6-trifluoromethylbenzenecyanide
[0039] 180g of 2-fluoro-3-chlorobenzotrifluoride was dissolved in 360mL of dry N,N-dimethylacetamide, the temperature was raised to 90°C, and 42.0g of sodium cyanide was slowly added in batches. After the addition, keep stirring at 90-100°C for 4 hours, and recover N,N-dimethylacetamide ...
Embodiment 2
[0046] 1) Preparation of 2-fluoro-3-chlorobenzotrifluoride
[0047] Add 215g of 2,3-dichlorobenzotrifluoride, 73g of potassium fluoride, 7g of triphenylphosphine bromide and 430mL of 1,3-dimethylimidazolidinone into a 1000mL three-necked flask equipped with a rectification column, and stir to raise the temperature Reflux reaction. After 1 hour of reaction, 185 g of a colorless transparent liquid began to evaporate slowly, and the content of GC analysis was 93.9%, which was 2-fluoro-3-chlorobenzotrifluoride. The yield of crude product is 93.2%. The crude product was directly used in the next reaction without purification.
[0048] 2) Preparation of 2-chloro-6-trifluoromethylbenzenecyanide
[0049] 180g of 2-fluoro-3-chlorobenzotrifluoride was dissolved in 360mL of dry N,N-dimethylacetamide, the temperature was raised to 90°C, and 42.0g of sodium cyanide was slowly added in batches. After the addition, keep stirring at 90-100°C for 4 hours, and recover N,N-dimethylacetamide ...
Embodiment 3
[0056] 1) Preparation of 2-fluoro-3-chlorobenzotrifluoride
[0057] Add 215g of 2,3-dichlorobenzotrifluoride, 83g of sodium fluoride, 10g of diisopropylethylamine and 400mL of N-methylpyrrolidone into a 1000mL three-necked flask equipped with a rectification column, stir and raise the temperature to reflux. After 4 hours of reaction, 183 g of a colorless transparent liquid began to evaporate slowly, and the content of GC analysis was 93.5%, which was 2-fluoro-3-chlorobenzotrifluoride. The yield of crude product is 92.2%. The crude product was directly used in the next reaction without purification.
[0058] 2) Preparation of 2-chloro-6-trifluoromethylbenzenecyanide
[0059] 180g of 2-fluoro-3-chlorobenzotrifluoride was dissolved in 360mL of dry dimethyl sulfoxide, the temperature was raised to 90°C, and 177.1g of potassium cyanide was slowly added in batches. After the addition, the reaction was refluxed for 2 hours. After vacuum distillation, the residue was dissolved in 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com