Low-reactivity inorganic boron nitride powder and preparation method thereof
A reactive, boron nitride powder technology, applied in inorganic chemistry, chemical instruments and methods, nitrogen compounds, etc., can solve problems such as unfavorable industrial utilization, low yield of solid polyborazane, etc. chemical application, shortening the preparation time, the effect of shortening the preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of low reactivity inorganic boron nitride powder, comprising the steps of:
[0030] S1) Add borazine into the autoclave, feed inert gas, and increase the pressure to 1MPa-5MPa; after increasing the pressure, the temperature is raised to 100-175°C for the first time, and the first holding time is 3.5-6h; Raise the temperature to 125-200°C for the second time, and the second holding time is 0.5-3h to obtain a solid product;
[0031] S2) The solid product is pulverized and refined to obtain inorganic boron nitride powder with low reactivity.
[0032] Specifically, the inert gas includes at least one of nitrogen and argon.
[0033] Specifically, the inert gas is argon.
[0034] Specifically, the temperature T of the first heating 1 and the temperature T of the second heating 2 meet T 2 = T 1 +25.
[0035] Specifically, the first holding time t 1 and the second holding time t 2 meet t 1 =t 2 +3
[0036] Specifically, argon gas was introduced,...
Embodiment 1
[0042]The preparation of sample 1 (inorganic boron nitride powder with low reactivity) includes the following steps:
[0043] Add 2kg of borazine into the autoclave, feed in nitrogen, and raise the pressure to 1MPa; after boosting the pressure, raise the temperature to 175°C for the first time and keep it for 6h; raise the temperature for the second time to 200°C and keep it for 3h to obtain a solid product ;
[0044] The solid product was pulverized and refined to obtain a low-reactivity inorganic boron nitride powder, that is, sample 1.
[0045] The yield of sample 1 was 1.85kg, and the yield was 92.6%.
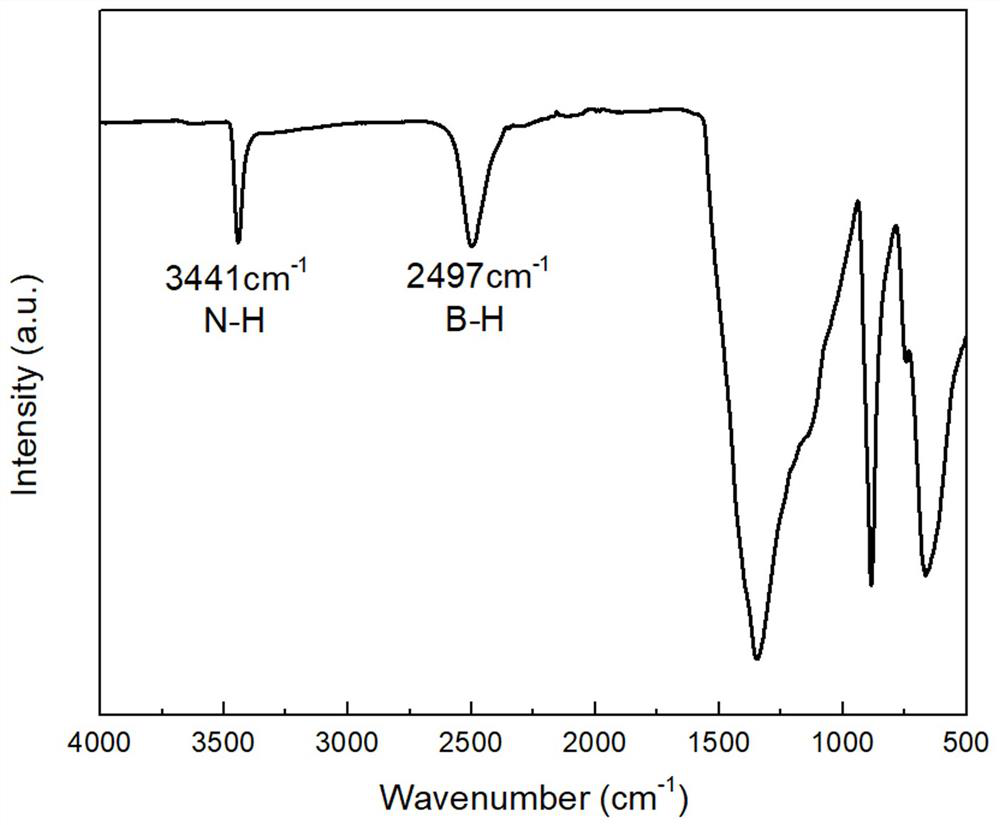
[0046] Grind sample 1 into powder, after grinding, carry out infrared scanning, such as figure 1 As shown, the infrared spectrum of sample 1 shows that there are stretching vibration peaks of B-H and N-H with weak absorption and small peak area, but at 1350cm -1 The strong and large-area absorption peak is the stretching vibration peak of B-N. Through the comparison of ...
Embodiment 2
[0050] The preparation of sample 2 (inorganic boron nitride powder with low reactivity) includes the following steps:
[0051] Put 4kg of borazine in a stainless steel iron box, then place it in an autoclave, blow in nitrogen, and raise the pressure to 3MPa; after boosting, raise the temperature to 125°C for the first time and keep it warm for 6h; raise the temperature to 150°C for the second time ℃, keep warm for 3h, obtain solid product;
[0052] The solid product was pulverized and refined to obtain a low-reactivity inorganic boron nitride powder, namely sample 2.
[0053] The yield of sample 2 was 3.72kg, and the yield was 93.1%.
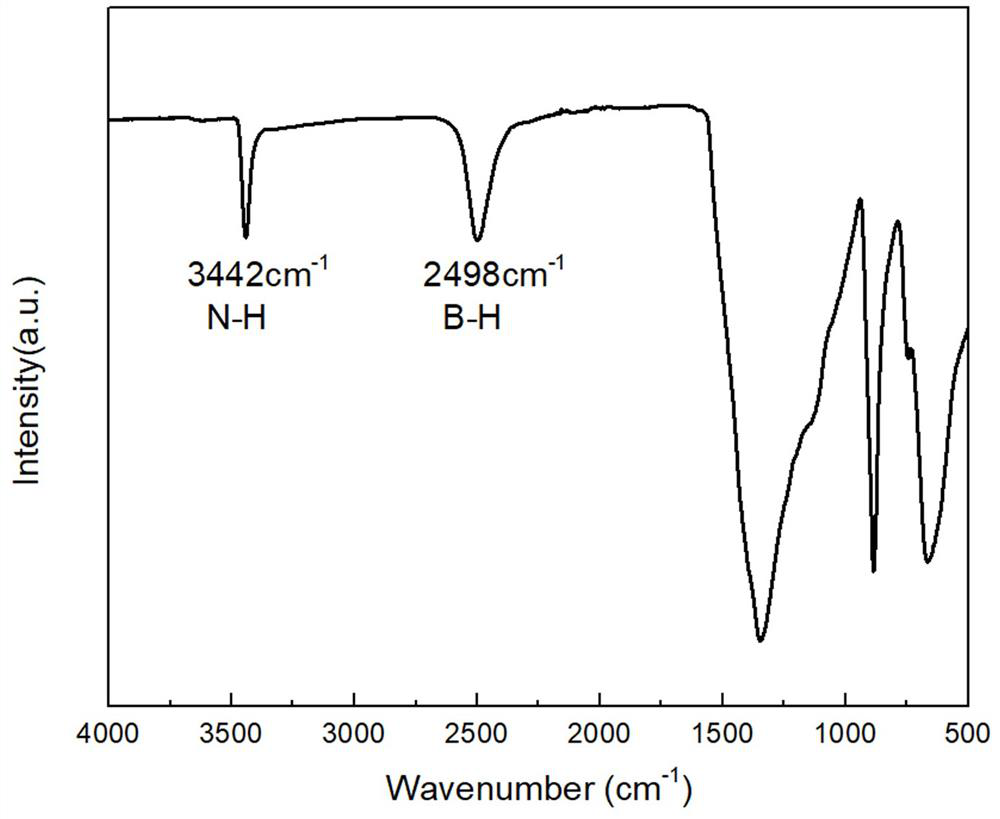
[0054] Grind sample 2 into powder, after grinding, carry out infrared scanning, such as figure 2 As shown, the infrared spectrum of sample 2 shows that there are stretching vibration peaks of B-H and N-H with weak absorption and small peak area, but at 1350cm -1 The strong and large-area absorption peak is the stretching vibration peak of B-N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com