Application of flufenamic acid as effective component in preparation of airway smooth muscle relaxation agent
A technology of airway smooth muscle and flufenamic acid, which is applied in the field of application of flufenamic acid as an active ingredient in the preparation of airway smooth muscle relaxants, can solve the problems of lack of powerful TAS2R agonists, and achieve broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Experimental object
[0043] Primary culture of human ASMCs
[0044] (2) Experimental equipment
[0045] Optical magnetic particle torsion cell analysis system (OMTC), live cell workstation, detachable 96-well plate, ultra-clean workbench, CO 2 Cell incubator, low-speed centrifuge, cell counting plate, constant temperature water bath, autoclave, confocal culture dish.
[0046] (3) Experimental process
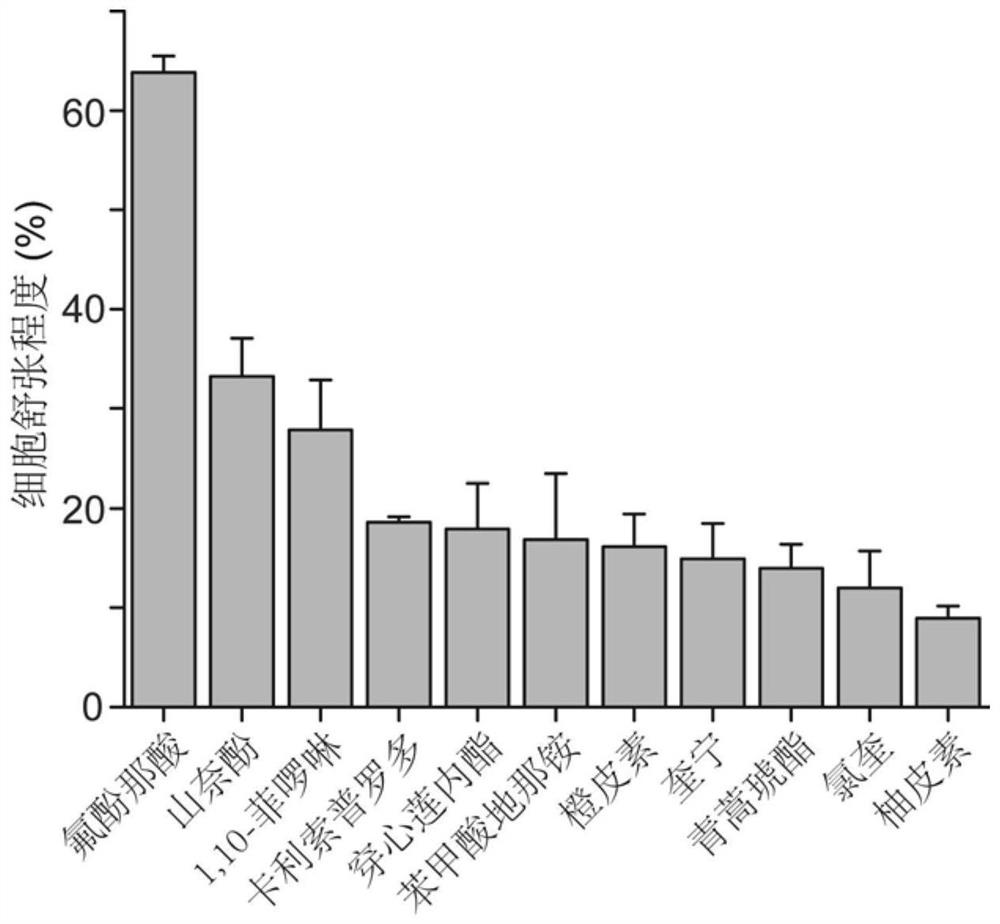
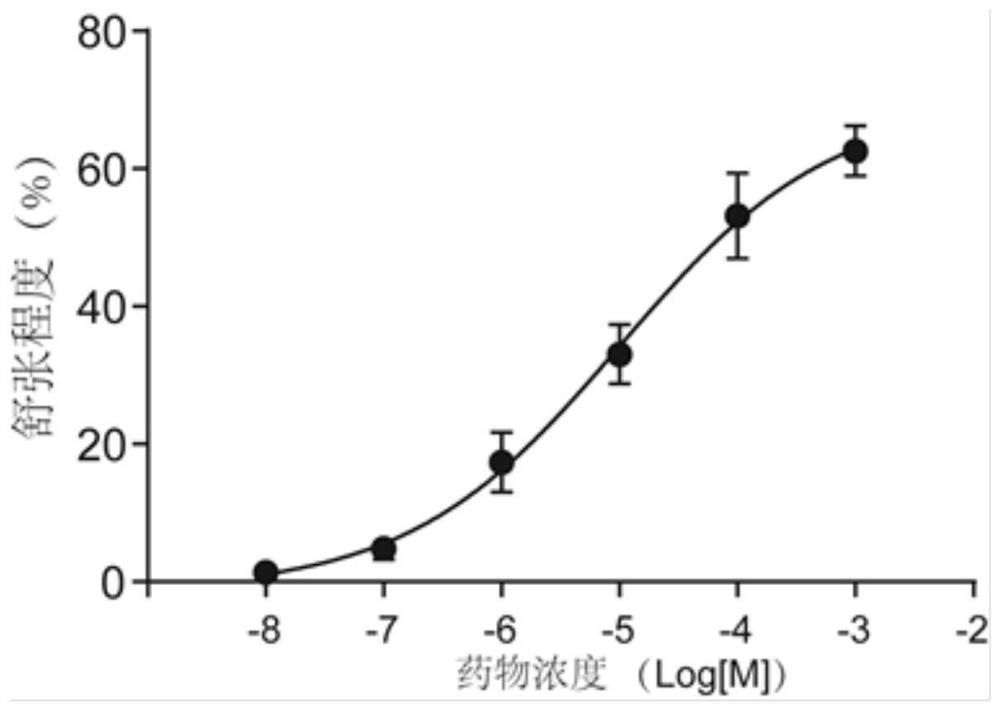
[0047] From the BitterDB database, 16 bitter substances (flufenamic acid, kaempferol, 1,10-phenanthroline, carlipidol, andrographolide, benzodinaridine) that can activate bitter taste receptors at concentrations below 10 μM were screened out. ammonium, hesperetin, quinine, chloroquine, naringenin, benzoin, tangeretin, apigenin, quercetin, cucurbitacin E, artesunate). After the cells were treated with the above 16 different bitter substances, the change of cell stiffness was detected by optical magnetic particle torsion cytometry, so as to evaluate the relaxation ...
Embodiment 2
[0056] (1) Experimental object
[0057] Primary culture of human ASMCs
[0058] (2) Experimental equipment
[0059] Optical magnetic particle torsion cell analysis system (OMTC), live cell workstation, detachable 96-well plate, ultra-clean workbench, CO 2 Cell incubator, low-speed centrifuge, cell counting plate, constant temperature water bath, autoclave, confocal culture dish.
[0060] (3) Experimental process
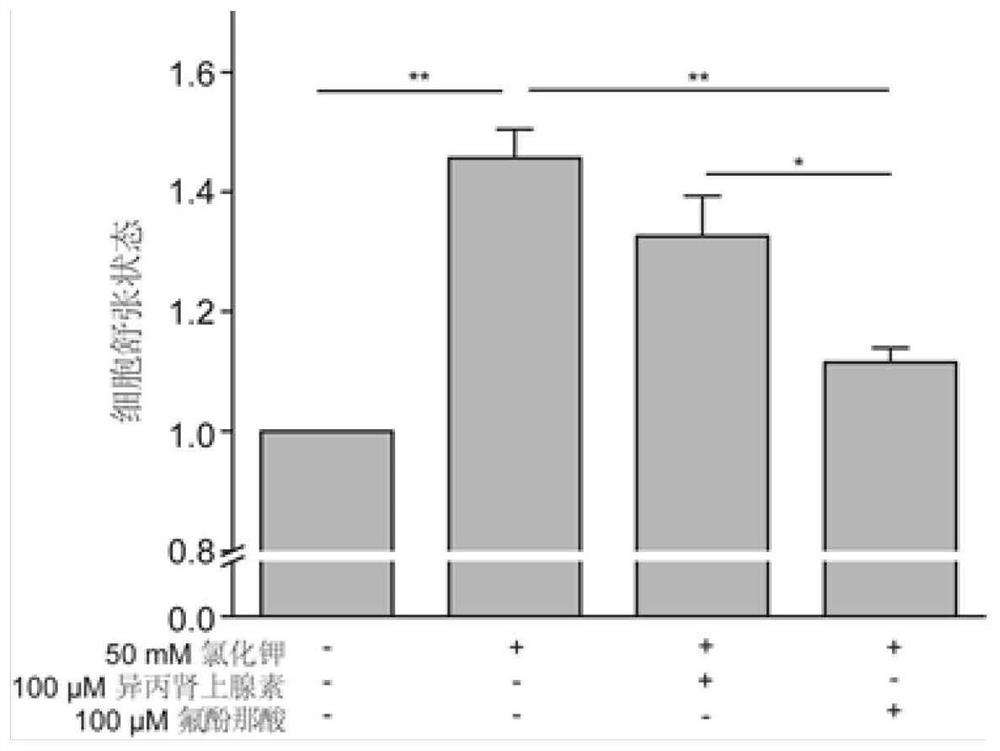
[0061] ASMCs were treated with flufenamic acid after inhibiting bitter taste receptors and downstream signaling pathways, and changes in cell stiffness were detected by optical magnetic particle torsion cytometry. The specific method is to inoculate the cells in a detachable 96-well plate at a seeding density of 1×10 4 After 24 hours of adherent growth, the IT medium was replaced for 12 hours, and the ASMCs were treated with siRNA targeting TAS2R14 for 48 hours to reduce the expression of bitter taste receptor 14, U73122 (20 μmol / L) to inhibit the activity of pho...
Embodiment 3
[0068] (1) Experimental object
[0069] BALB / c mouse
[0070] (2) Experimental equipment
[0071] FlexiVent small animal lung function measuring instrument, ultra-clean workbench, constant temperature water bath.
[0072] (3) Experimental process
[0073] The airway resistance of normal and asthma model mice was detected by FlexiVent small animal pulmonary function measuring instrument, and the airway diastolic function of flufenamic acid was evaluated. First, the animals were randomly divided into 6 groups: normal control group, asthma model group (ovalbumin treatment) control group, flufenamic acid high, medium and low dose groups (8mg / kg, 4mg / kg, 2mg / kg) And albuterol group (8mg / kg), 5 rats in each group.
[0074] The process of establishing the asthma model was to inject 200 μL ovalbumin sensitizer into each mouse intraperitoneally on the 1st and 7th day, and give 100 μL ovalbumin sensitizer to the intraperitoneal injection on the 14th day; the normal control group was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com