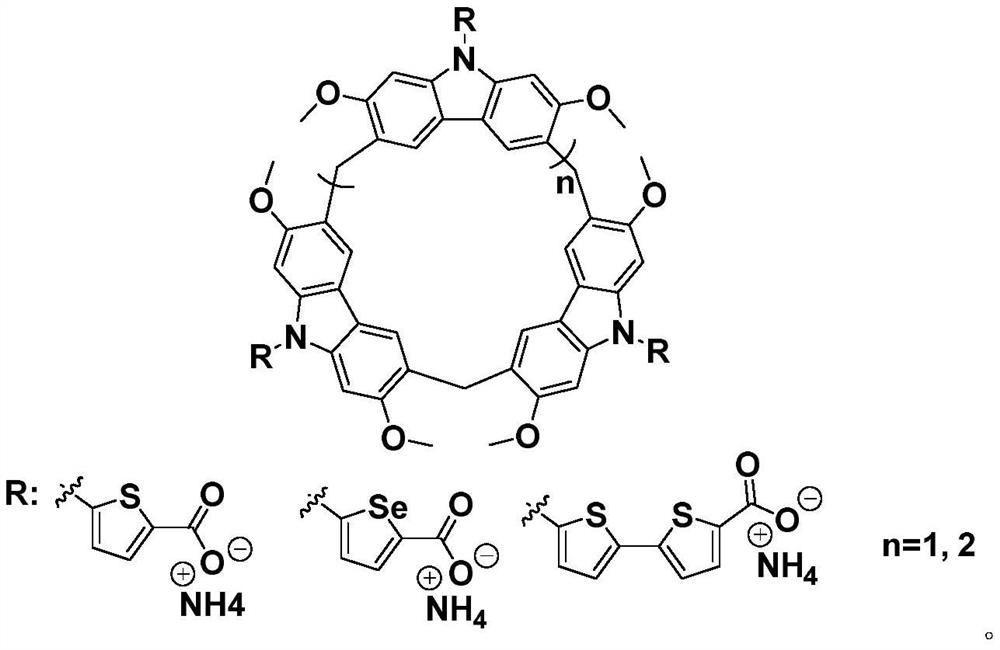
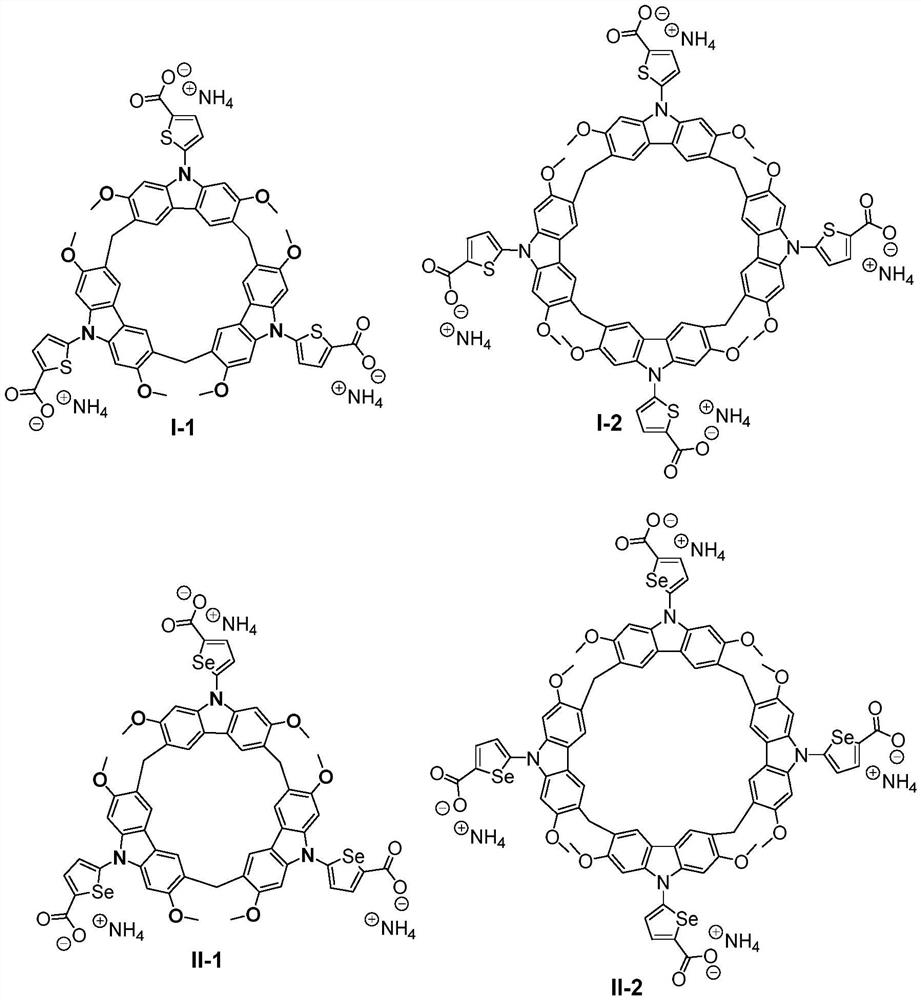
Substituted calix carbazole derivative as well as synthesis method and application thereof
A technology of carbazole derivatives and derivatives, which is applied in the field of medical technology and can solve the problems of berberine hydrochloride adverse reactions and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Dissolve 2.06g of 5-bromothiophene-2carboxylic acid in sulfur oxychloride, reflux for 5h, remove the solvent under reduced pressure, add toluene solution, 1.8g of triethylene glycol monomethyl ether and pyridine, reflux for 6h, then remove under reduced pressure solvent, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and finally purified by column chromatography to obtain compound A-1.
[0022]
[0023] Dissolve 3.52g of compound A-1 and 2.7g of 2,7-dimethoxy-9H-carbazole in DMF, then add cuprous iodide and potassium carbonate, react at 130°C for 3h, then remove under reduced pressure solvent, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and finally purified by column chromatography to obtain compound B-1.
[0024] Dissolve 1 g of compound B-1 in dichloromethane, add paraformaldehyde (0.09 g, 3 mmol) and 0.27 g of ferric chloride, react at 27 ° C for 3-4 h, add ammonia water to quench, and then extract, The solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com