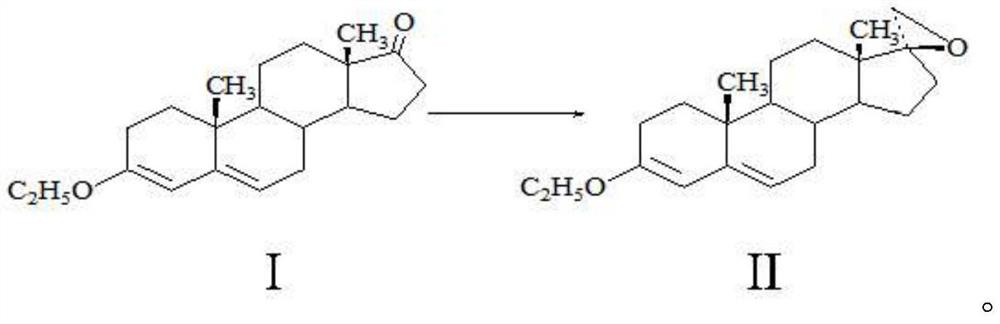
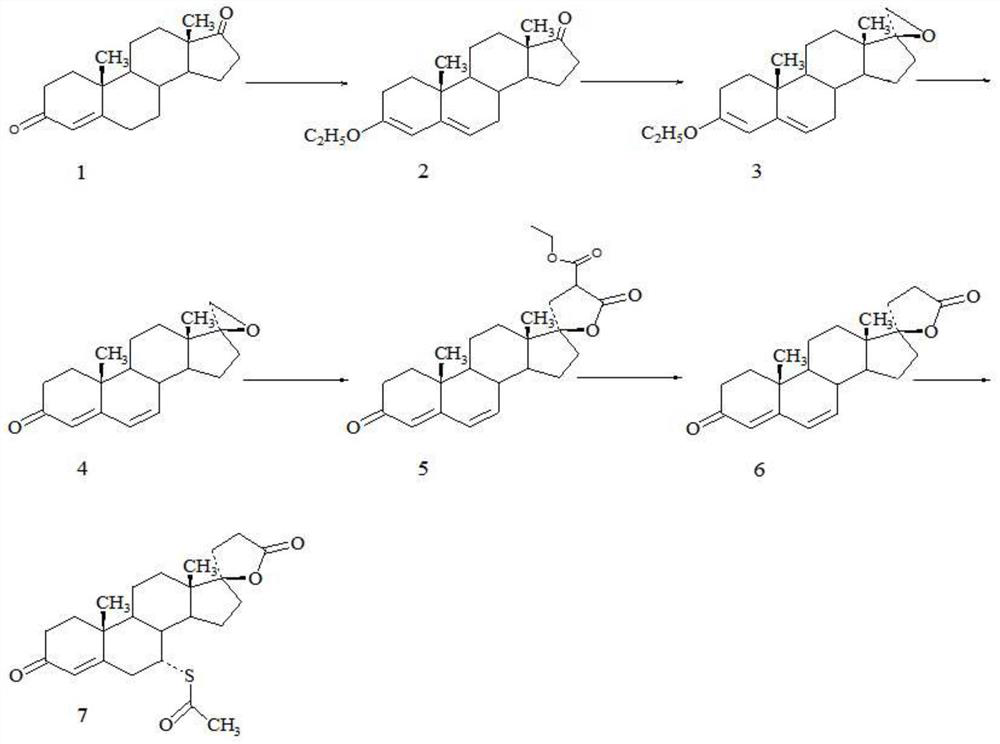
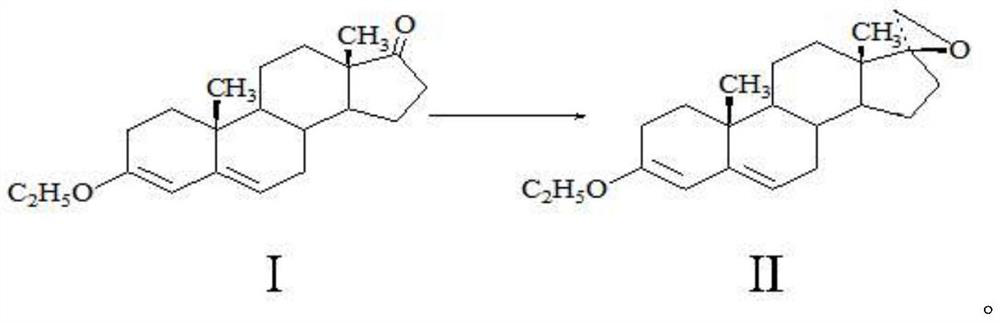
Method for preparing spirolactone key intermediate epoxide
A technology of bulk epoxy and spironolactone, which is applied in the field of drug synthesis, can solve problems such as high price of strong organic bases, troubles in environmental protection work, difficult removal of filtrate, etc., and achieve the effects of reducing power costs, reducing energy consumption, and being easy to recycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A method for preparing spironolactone key intermediate epoxy, comprising the following steps: 20g of ether compound I is dissolved in 100ml of dichloromethane, 2ml of 1-butyl-3-methylimidazolium tetrafluoroborate, 20g of Trimethylsulfonium bromide, 100ml of sodium hydroxide solution with a mass fraction of 20%, stirred and reacted at 40°C for 5 hours, separated the water layer, and concentrated the organic phase to a small volume under normal pressure (the amount of solvent is about 50-60ml), cooling, 0 It was kept at -10°C for 2 hours, and 17.8 g of solid was obtained by filtration. After testing: the melting point of the obtained product is 104-106° C. (literature value 105° C.), the molar yield is 85.21%, the NMR test confirms that it is epoxy compound II, and the HPLC test shows that its purity is 99.2%.
Embodiment 2
[0021] A method for preparing spironolactone key intermediate epoxy, comprising the following steps: 20g of ether compound I is dissolved in 400ml of toluene, 20ml of 1-methyl-3-methylimidazolium tetrafluoroborate, 20g of brominated Trimethylsulfonium, 30ml of potassium hydroxide solution with a mass fraction of 30%, stirred and reacted at 20°C for 5 hours, separated the water layer, and concentrated the organic phase under reduced pressure (absolute pressure not greater than 0.02MP, temperature less than 80°C) to a small volume (The amount of solvent is about 50-60ml), lower the temperature, keep warm at 0-10°C for 2 hours, and filter to obtain 18.2g of solid. After testing: the melting point of the obtained product is 104-105°C (literature value 105°C), the molar yield is 87.12%, the NMR detection is determined to be epoxy II, and the HPLC detection has a purity of 99.0%.
Embodiment 3
[0023] A method for preparing spironolactone key intermediate epoxy, comprising the following steps: 20g of ether compound I is dissolved in 200ml of methyl tetrahydrofuran, and 30ml of 1-methyl-3-ethylimidazole tetrafluoroborate, 20g Trimethylsulfonium bromide, 200ml of 50% mass fraction of sodium hydroxide solution, stirred and reacted at 10°C for 5 hours, separated the water layer, and concentrated the organic phase under reduced pressure (absolute pressure not greater than 0.02MP, temperature less than 60°C) to Small volume (solvent amount about 50-60ml), lower the temperature, keep warm at 0-10°C for 2 hours, lower the temperature, and filter to obtain 18.1g of solid. After detection: the melting point of the obtained product is 105-106° C. (literature value 105° C.), the molar yield is 86.64%, and the NMR detection is determined to be epoxy compound II, and the HPLC detection has a purity of 99.3%.
[0024] The part characteristic hydrogen data of embodiment 1-3 gained p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com