Construction method of human embryonic stem cells
A technology for human embryonic stem cells and construction methods, which can be used in microorganism-based methods, chemical instruments and methods, and botanical equipment and methods, etc., and can solve the problem of immune rejection without a good solution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
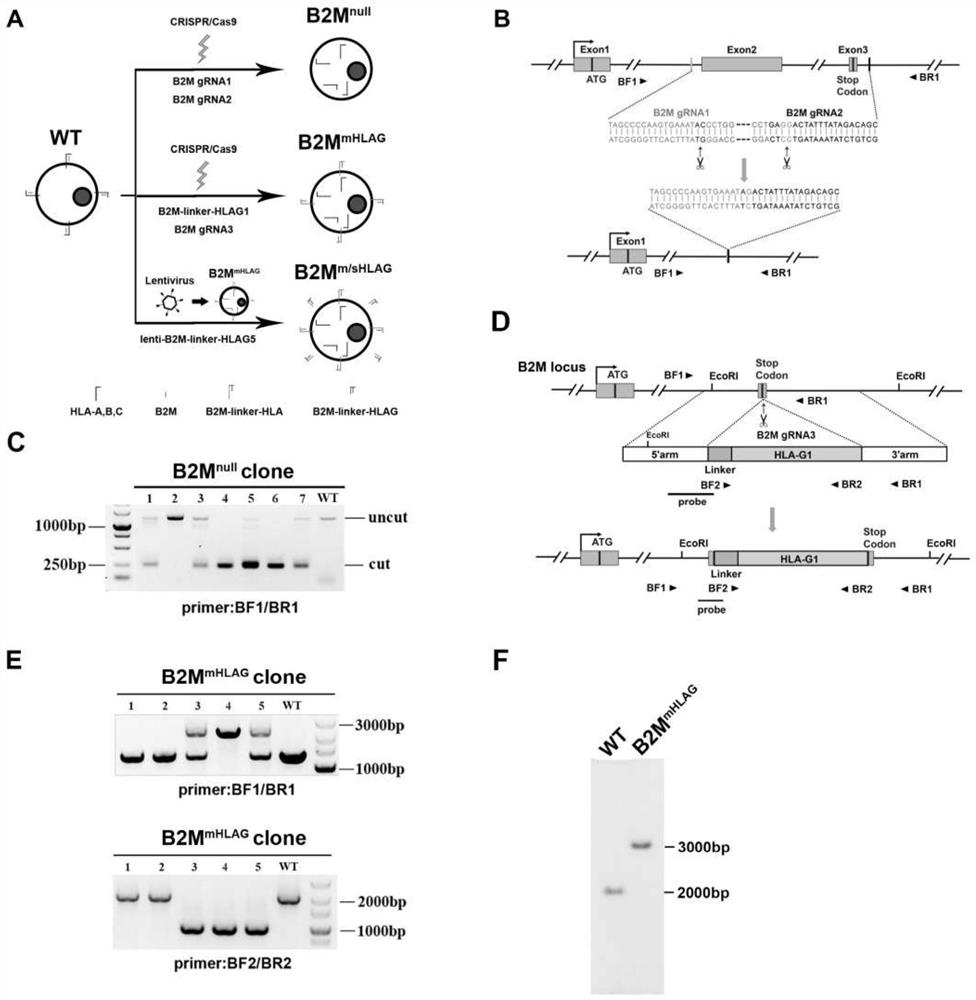
[0059] Construction of hESCs cell line with biallelic B2M gene deletion by dual gRNA gene editing system
[0060] The dual gRNA gene editing strategy can efficiently and specifically knock out biallelic genes, and the cut DNA is mainly repaired by direct end joining without introducing insertion or deletion of nucleotides (Liu et al., 2016). To knock down the B2M gene in human embryonic stem cells (hESCs), two adjacent gRNAs were designed, one located upstream of exon 2 of the B2M gene and the other downstream of exon 3 ( figure 1 A and figure 1 B), the specific sequences are respectively shown in B2M gRNA 1 and B2M gRNA 2 in Table 1. The vast majority of mature B2M proteins are encoded by this targeting sequence.
[0061] Table 1
[0062]
[0063] Two gRNA vectors (Addgene #41824), CAG promoter-driven Cas9-P2A-GFP vector (Addgene #44719) and a vector for transient expression of puromycin (SEQ ID NO.14) were co-electrotransfected into H9 hESC (generated by Provided by P...
Embodiment 2
[0065] Construction of hESCs cell lines expressing HLA-G1 and HLA-G5
[0066] Two hESCs cell lines expressing HLA-G protein were designed ( figure 1 A). In B2MmHLAG hESCs, the HLA-G1 heavy chain is flexible (G 4 S) 4 The connecting peptide is fused to endogenous B2M protein. In order to use the coding HLA-G1 heavy chain reading frame and flexible (G 4 S) 4 The sequence of the connecting peptide replaces the stop codon located in exon 3 of the endogenous B2M gene, and the B2M gRNA3 (see Table 1) that spans the B2M stop codon is designed, so that the gene locus that is successfully knocked in will not be detected by B2M gRNA3 Combined, thereby improving the efficiency of establishing homozygous knock-in cell lines. Cas9 vector driven by CAG promoter (same as Example 1), recombinant donor (SEQ ID No.9), B2M gRNA3 vector (Addgene #41824) and transient expression puromycin vector (same as Example 1) were electroporated together into H9 hESCs. In this way, (G 4 S) 4 -HLA-G...
Embodiment 3
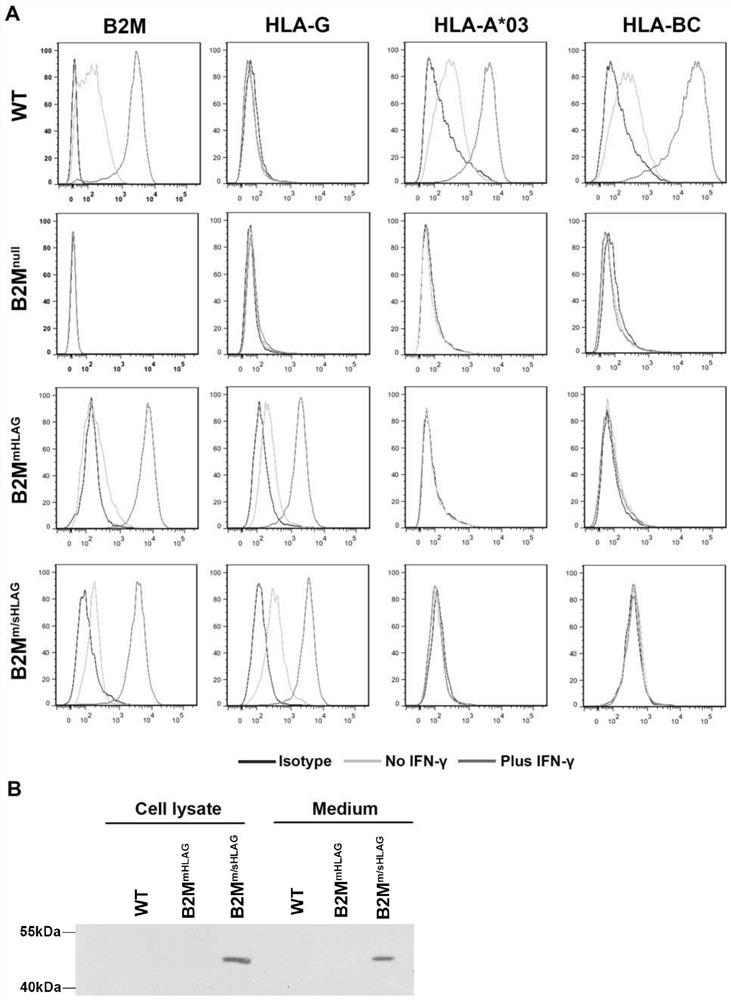
[0092] Detection of HLA I protein expression in genetically modified cell lines
[0093] To study the expression of β2m and HLA class I proteins in wild-type (WT), B2M null , B2M mHLAG and B2M m / sHLAGThe expression in hESCs cell lines was performed by Western Blot and flow cytometry (FACS) experiments. Before the flow cytometry experiment, IFN-γ was added to the hESCs cell culture medium to a final concentration of 25 ng / ml, and after 48 hours of culture, the flow cytometry experiment was performed. In case of IFN-γ stimulation, wild type, B2M mHLAG and B2M m / sHLAG After hESCs were treated with IFN-γ, the expression of β2m protein on the cell membrane surface was significantly increased ( image 3 A), demonstrating that the integration of HLA-G1 in the B2M gene does not affect the expression of endogenous B2M. However, even under IFN-γ stimulation, in B2M null The cell membrane surface expression of β2m protein could not be detected in hESCs ( image 3 A). Thus, it wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com