Triazole derivatives capable of affecting calcium ion channels of tumor cells, preparation method and application thereof
A calcium ion channel, tumor cell technology, applied in the field of triazole derivatives and their preparation, can solve the problems of patients being susceptible to various tumors, reducing human immunity, etc., and achieve significant application value, improve yield, and improve response. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
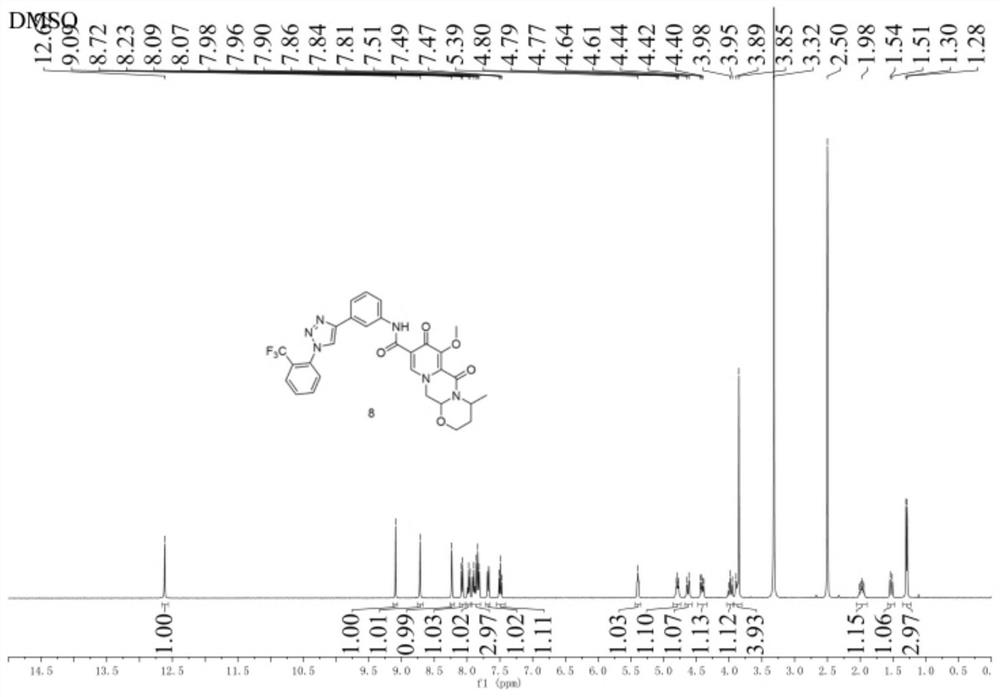
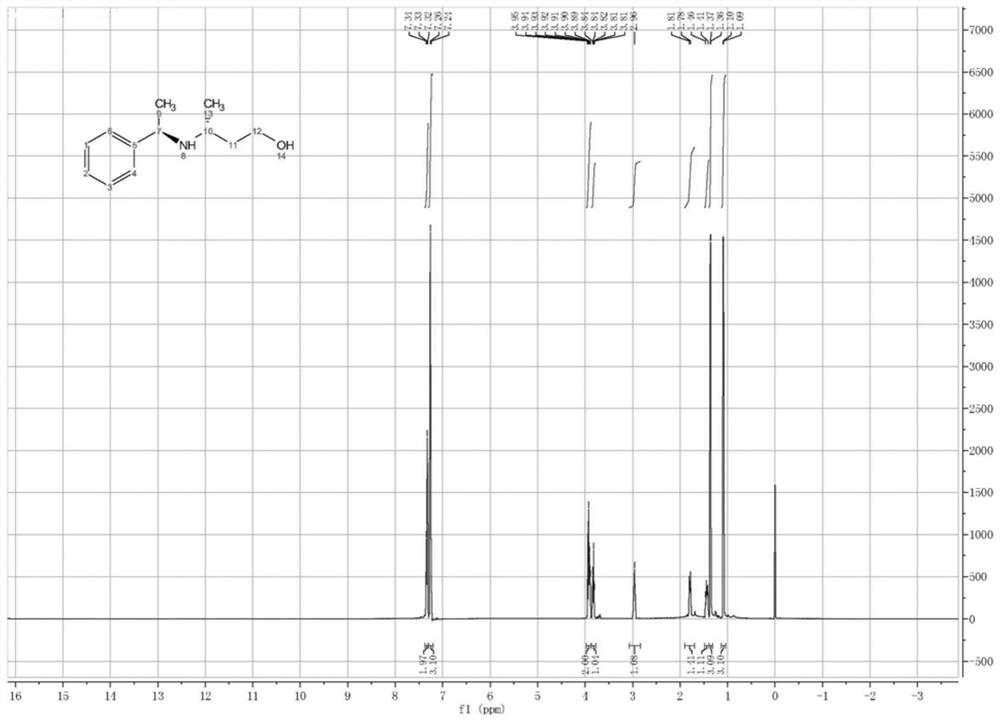
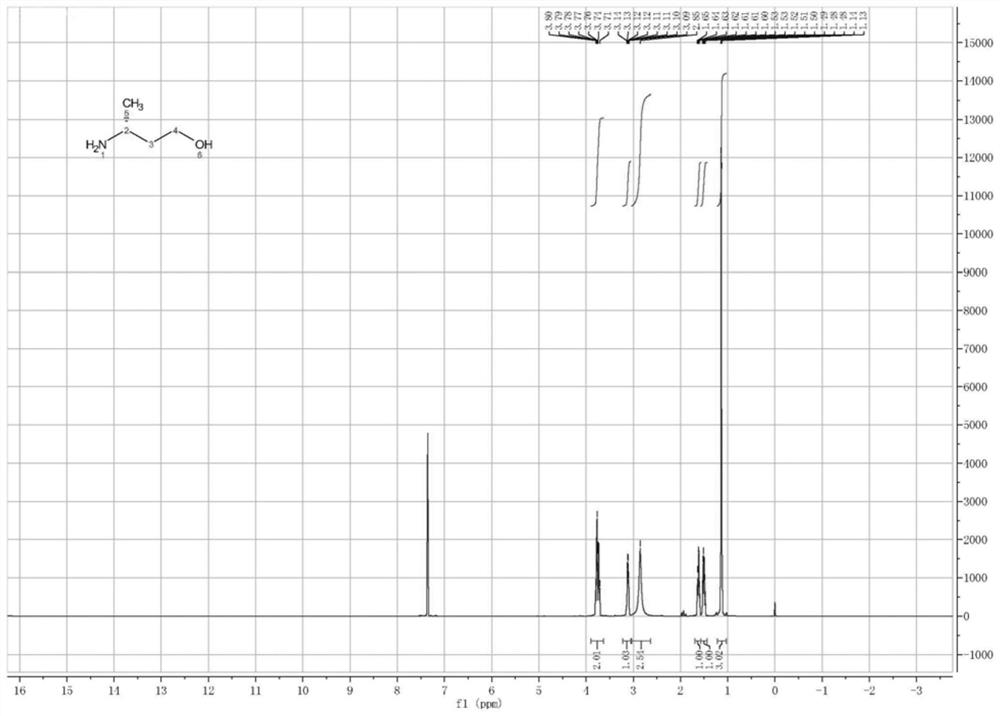
Image
Examples
Embodiment 1
[0076]
[0077] Under the protection of nitrogen and the reaction temperature at 15-25°C, add 30 g of methyl 4-methoxyacetoacetate into 100 mL of tetrahydrofuran, add 24 g of N,N-dimethylformamide dimethyl acetal dropwise, After the addition, keep the temperature at room temperature, stir the reaction for 1 h, then raise the temperature to 35°C, and concentrate to obtain (Z)-2-((2,2-methylamino)methylene)-4-methoxy- Methyl 3-oxobutanoate 37g, LC-MS: 202[M+H] + .
Embodiment 2
[0079]
[0080] Add 20g of (Z)-2-((2,2-methylamino)methylene)-4-methoxy-3-oxobutanoic acid methyl ester to 250mL of anhydrous methanol, at room temperature and nitrogen protection Stir under low temperature until completely dissolved; add 48g of dimethyl oxalate, then cool down to 10°C, add 12g of sodium methoxide, heat up to reflux, cool down to 0°C after the reaction of the raw materials is complete, add dropwise 2N hydrochloric acid to adjust the pH value of the reaction solution to 5-6 , then concentrated in vacuo, then added 150 mL of ethyl acetate to the concentrate, separated the organic phase and then concentrated it into 200 mL of anhydrous methanol, then added 11 g of aminoacetaldehyde dimethyl acetal, stirred for 2.5 hours, cooled to 5°C, added Anhydrous lithium hydroxide 7.2g, control the reaction between 0-5°C; after 30 minutes of reaction, TLC shows that the raw materials have reacted completely, keep the reaction temperature at 0-5°C, add dilute sulfuric acid ...
Embodiment 3
[0082]
[0083] Add 20g of (Z)-2-((2,2-methylamino)methylene)-4-methoxy-3-oxobutanoic acid methyl ester to 250mL of anhydrous methanol, at room temperature and nitrogen protection Stir under low temperature until completely dissolved; add 48g of dimethyl oxalate, then cool down to 10°C, add 15g of sodium ethoxide, heat up to reflux, cool down to 0°C after the reaction of the raw materials is complete, add 2N hydrochloric acid dropwise to adjust the pH value to 5-6, and then Concentrate in vacuo, then add 150 mL of ethyl acetate to the concentrate, separate the organic phase and concentrate, add to 200 mL of anhydrous methanol, then add 11 g of aminoacetaldehyde dimethyl acetal, stir for 2.5 hours, cool to 5 ° C, add anhydrous Lithium hydroxide 7.2g, control the reaction at 0-5°C; after reacting for 30 minutes, keep the reaction temperature at 0-5°C, add 2N hydrochloric acid solution dropwise to adjust the pH value to 1-2, add 100mL of dichloromethane, and separate the organi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com