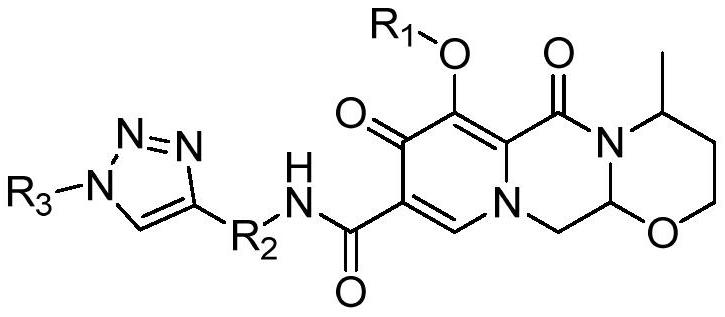
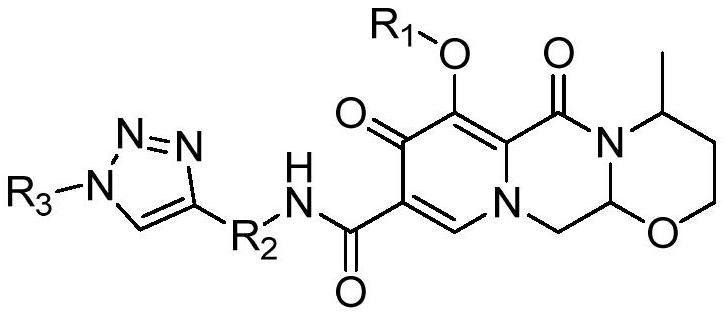
Dolutegravir derivative with biological activity as well as preparation method and application thereof
A technology of biological activity and derivatives, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of reducing human immunity and susceptibility of patients to various tumors, so as to achieve method optimization, increase yield, and expand application range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068]
[0069] Add compound 1 (30g) into 100mL of anhydrous formic acid, and heat to 65°C under the protection of argon; after reacting for about 3 hours, add 150mL of acetonitrile after concentration, stir to dissolve, add 12.5g of R-3-aminobutanol, Stir for 10 minutes; raise the temperature to reflux and stir for 2 hours; add 200 mL of dichloromethane after vacuum concentration and steaming, add 100 mL of water while stirring, then add 2N hydrochloric acid to adjust the pH of the reaction solution to 1-2, separate the lower organic phase after stirring for 10 minutes, and separate the upper layer of water The phase was extracted three times with 50 mL of dichloromethane, and the combined organic phases were concentrated to obtain compound 2 (25.5 g); 1 H NMR (400MHz, CDCl 3 ):δ8.43(s,1H),5.30(t,J 1 =4.0Hz,J 2 =4.0Hz,1H),5.02(t,J 1 =4.0Hz,J 2 =8.0Hz,1H),4.41(dd,J 1 =4.0Hz,J 2 =4.0Hz,1H),4.27(dd,J 1 =8.0Hz,J 2 =4.0Hz,1H),4.08(s,3H),4.03-3.99(m,2H),2.25-2.16(m,1H),1...
Embodiment 2
[0071]
[0072] Add compound 1 (30g) into a mixture of 70mL of anhydrous formic acid and 40mL of anhydrous acetic acid, and heat to 65°C under the protection of argon; -12.5g of 3-aminobutanol, stirred for 10min; heated to 80°C and stirred for 8h; after vacuum concentration and steaming, 200mL of dichloromethane was added, and 100mL of water was added under stirring, and the lower organic phase was separated after stirring for 10min, and the upper aqueous phase was washed with distilled water Extracted three times with 50 mL of methyl chloride, combined the organic phases, and concentrated to obtain pure compound 2 (23.1 g); 1 H NMR (400MHz, CDCl 3 ):δ8.43(s,1H),5.30(t,J 1 =4.0Hz,J 2 =4.0Hz,1H),5.02(t,J 1 =4.0Hz,J 2 =8.0Hz,1H),4.41(dd,J 1 =4.0Hz,J 2 =4.0Hz,1H),4.27(dd,J 1 =8.0Hz,J 2=4.0Hz,1H),4.08(s,3H),4.03-3.99(m,2H),2.25-2.16(m,1H),1.56(d,J=12.0Hz,1H),1.39(d,J= 8.0Hz, 3H).
Embodiment 3
[0074]
[0075] Add compound 1 (30g) and 24g of benzenesulfonic acid into 300mL of acetonitrile, heat to 70°C under the protection of argon; after reacting for about 5h, add 18g of R-3-aminobutanol, stir for 10min; heat up to 80°C and stir the reaction 3.5h; add 200mL of dichloromethane after vacuum concentration and steaming, add 150mL of water under stirring, separate the lower organic phase after stirring for 10min, extract the upper layer of water phase with 50mL of dichloromethane three times, combine the organic phases, wash with 40mL of saturated saline for 3 Then concentrated to obtain the crude product, finally recrystallized and purified in methanol to obtain pure compound 2 (19.1g); 1 H NMR (400MHz, CDCl 3 ):δ8.43(s,1H),5.30(t,J 1 =4.0Hz,J 2 =4.0Hz,1H),5.02(t,J 1 =4.0Hz,J 2 =8.0Hz,1H),4.41(dd,J 1 =4.0Hz,J 2 =4.0Hz,1H),4.27(dd,J 1 =8.0Hz,J 2 =4.0Hz,1H),4.08(s,3H),4.03-3.99(m,2H),2.25-2.16(m,1H),1.56(d,J=12.0Hz,1H),1.39(d,J= 8.0Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com