Total synthesis method of bepotastine besilate
A synthetic method, the technology of bepotastine besilate, which is applied in the field of total synthesis of bepotastine besilate, can solve the problems of low yield, harsh reaction conditions, high energy consumption, etc., and achieve cheap and easy-to-obtain raw materials, mild reaction conditions, The effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A kind of bepotastine besilate total synthesis method, comprises the following steps:
[0052] (1) Preparation of compound 2
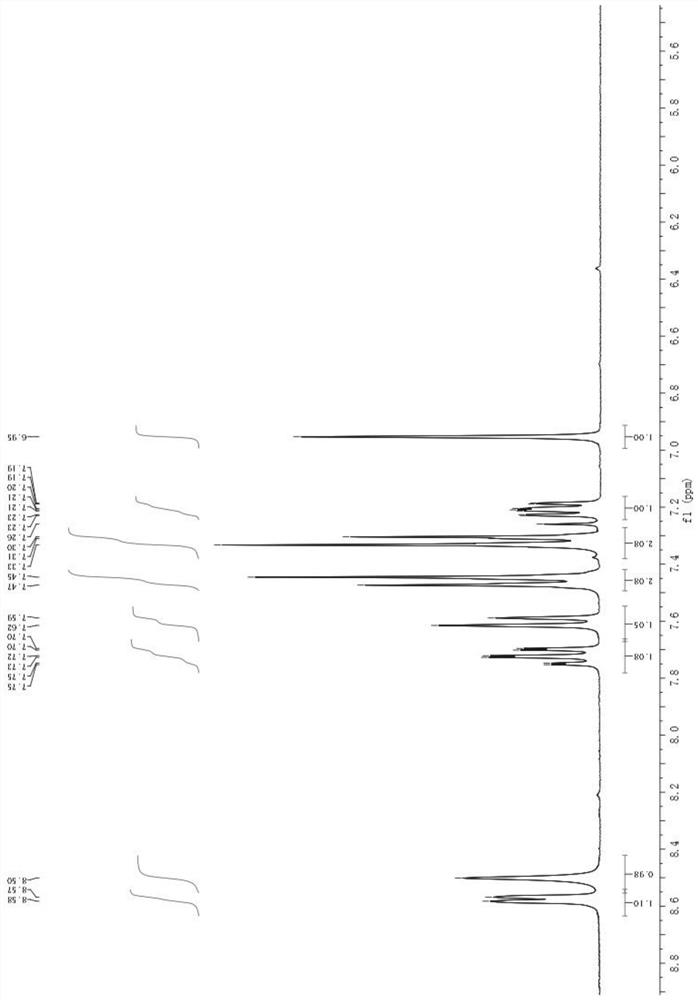
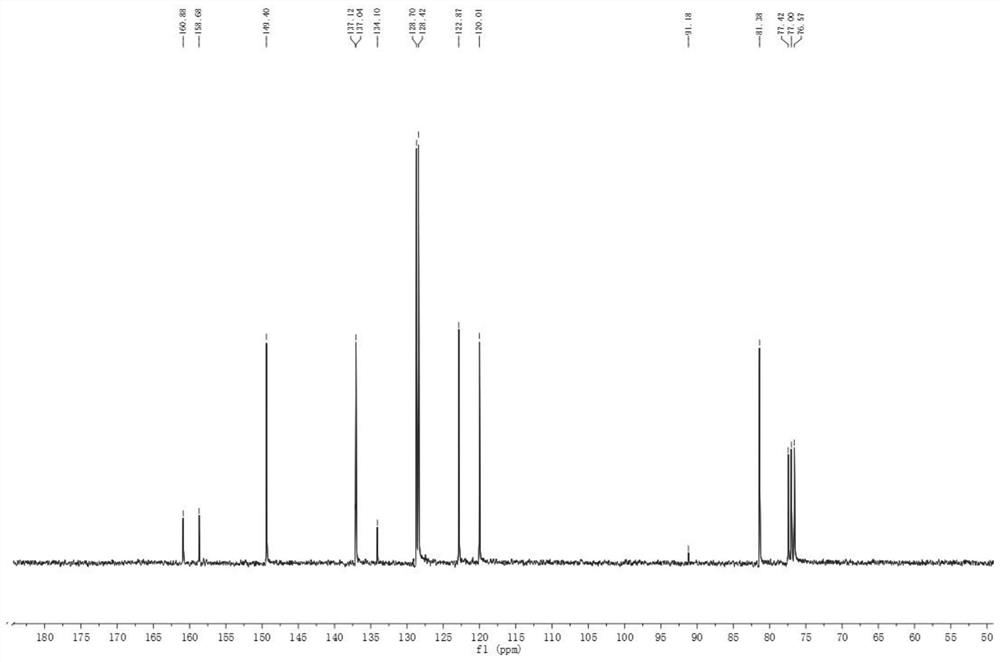
[0053] Add 88g of (4-chlorophenyl)(2-pyridyl)-methanol and 880g of dichloromethane into the reaction flask, and lower the temperature to 0-5°C. Add 22.4g of KOH, slowly add 115.6g of trichloroacetonitrile dropwise, control the reaction temperature below 5°C, after dropping, keep warm for 10 minutes, add 400g of water dropwise, stir and separate the liquid, and concentrate the organic phase to dryness under reduced pressure to obtain 142.7g of compound 2. The yield is 98.0%; its hydrogen spectrum and carbon spectrum are as follows figure 1 , 2 shown;
[0054] (2) Preparation of Compound 3
[0055] Add 124g of N-ethoxycarbonyl-4-hydroxypiperidine and 2kg of dichloromethane into the reaction flask. When the temperature is lowered to about 10°C, add 200g of compound 2. After compound 2 is completely dissolved, add 84g of trifluoromethanesulfonic...
Embodiment 2
[0067] A kind of bepotastine besilate total synthesis method, comprises the following steps:
[0068] (1) Preparation of Compound 2
[0069] Add 61.5g of (4-chlorophenyl)(2-pyridyl)-methanol and 615g of diethyl ether into the reaction flask, and cool down to 0-5°C. Add 31.42 g of potassium tert-butoxide, slowly add 80.86 g of trichloroacetonitrile dropwise, control the reaction temperature below 5°C, after the drop is completed, keep the temperature for 10 minutes, add 300 g of water dropwise, stir and separate the liquids, and concentrate the organic phase to dryness under reduced pressure to obtain 91.1g compound 2, yield 89.5%;
[0070] (2) Preparation of Compound 3
[0071] Add 62 g of N-ethoxycarbonyl-4-hydroxypiperidine and 1 kg of chloroform into the reaction flask. When the temperature is lowered to -10°C, add 100 g of compound 2. After compound 2 is completely dissolved, add 39.0 g of boron trifluoride diethyl ether dropwise to control the reaction. The temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com