Peptides and pharmaceutical compositions for treating eye diseases
A compound, alkyl technology, applied in the field of peptides and pharmaceutical compositions for the treatment of eye diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
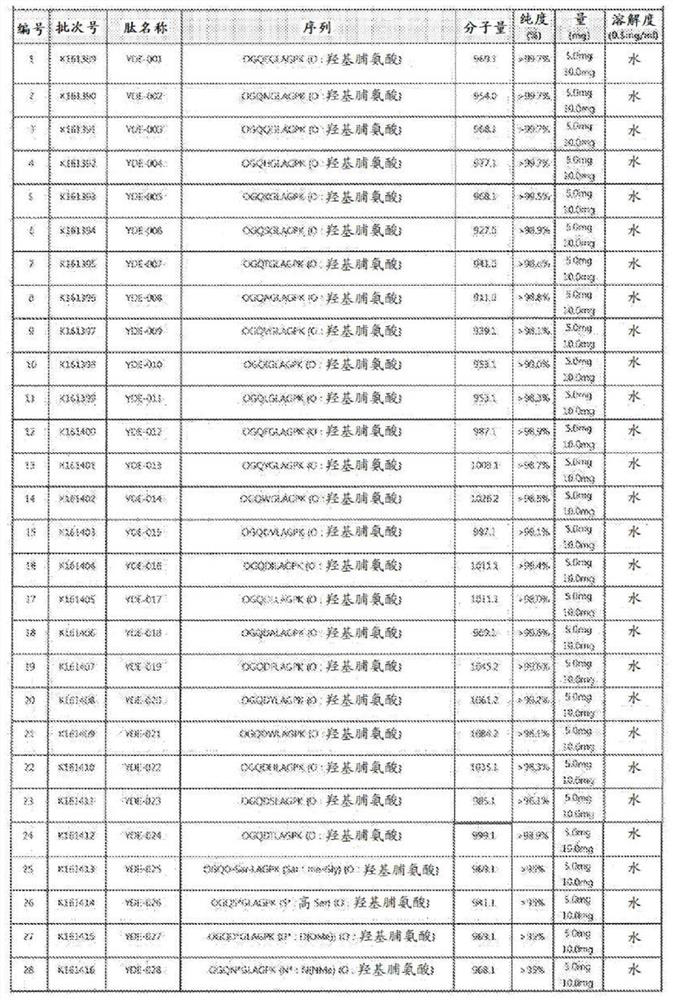
[0860] Embodiment 1: the preparation of YDE derivative
[0861] Protein analysis of the extracellular matrix derived from animal chondrocytes was performed in Baek's group of Center of Biomedical Mass Spectrometry (Diatech Korea Co., Ltd., Seoul, Korea). Proline-GQDGLAGPK (P-GQDGLAGPK), which is a part of the amino acid sequence of type II collagen α1 protein, was obtained through the protein analysis described above.
[0862] The following is an exemplary protein synthesis of YDE-011. Other compounds of the invention (e.g., YDE-001-YDE-086) were prepared via similar procedures, e.g., by substituting different amino acid building block reagents at the desired steps.
[0863]
[0864] Exemplary program for YDE-011
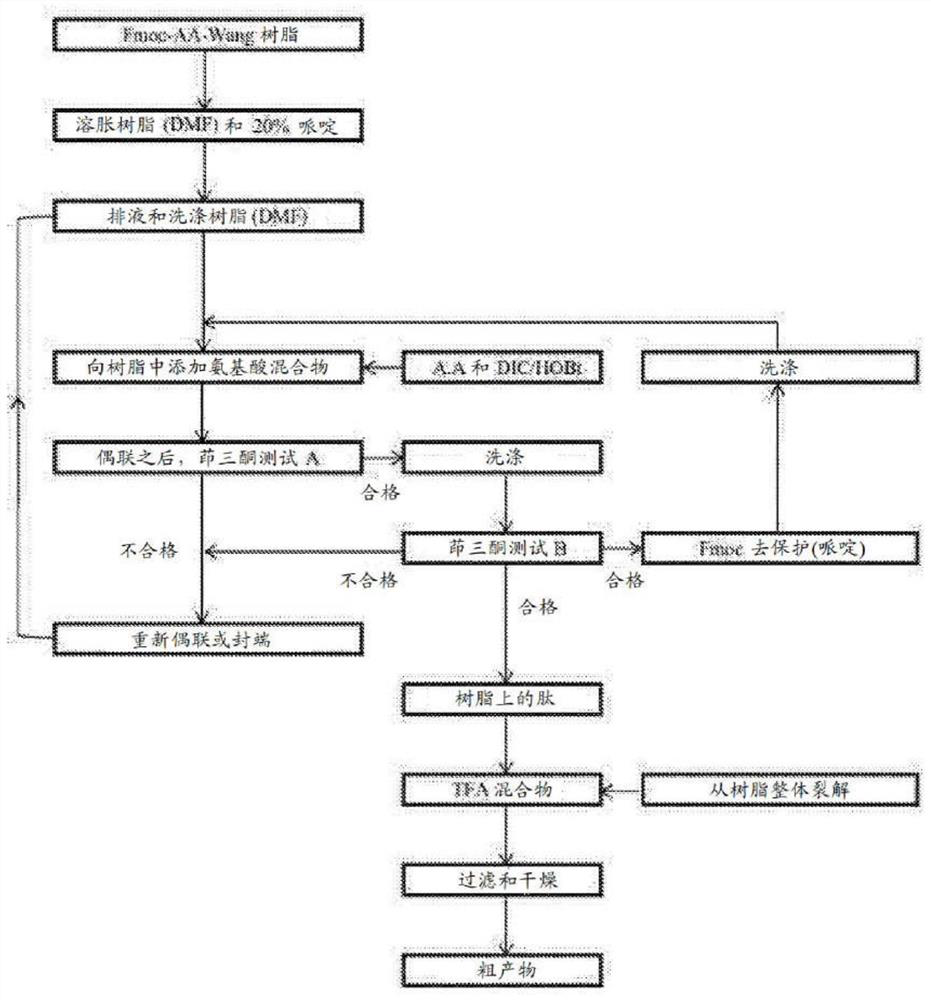
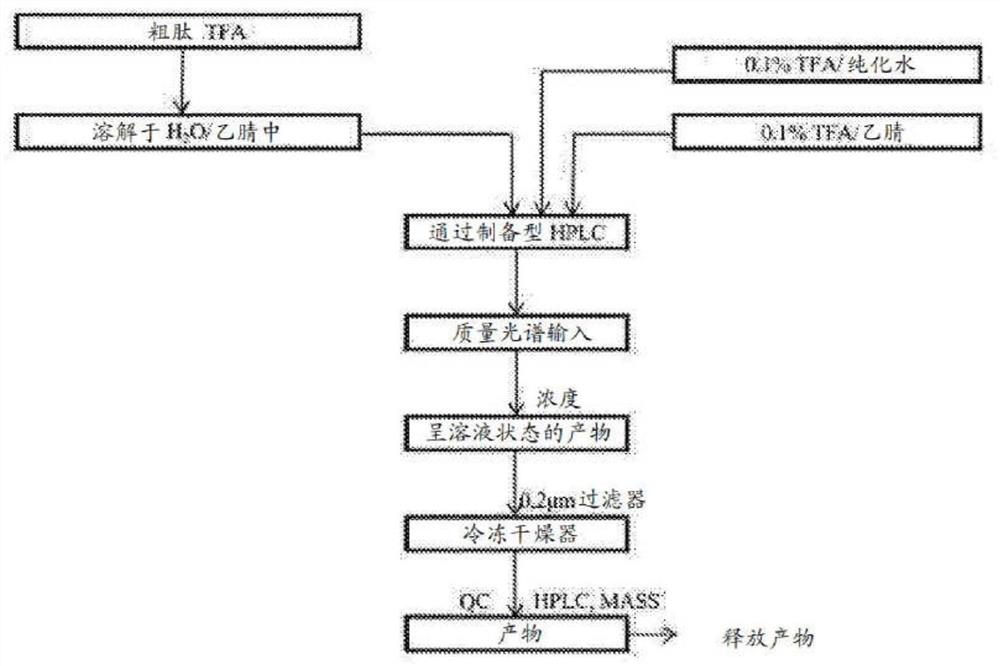
[0865] solid phase synthesis
[0866] Fmoc-Pro-Lys(Boc)-Wang Resin(1)
[0867]To a solid phase synthesis reactor equipped with a filter membrane was added Fmoc-Lys(Boc)-Wang resin (1.75 g, 1 mmol) in DCM (30 mL), followed by swelling for 30 min, followed by...
Embodiment 2
[0911] Example 2: Preparation of YDE derivatives with modified C-terminus
[0912] Preparation of YDE peptide
[0913] YDE peptides (YDE-093, YDE-096 and YDE-101 to YDE-107), derivatives of the amino acid sequence of YDE-011 were obtained via C-terminal modification of YDE peptides such as YDE-011.
[0914] To prepare C-terminal modified peptides, Fmoc solid-phase peptide synthesis (SPPS) was performed based on the standard procedure described in WO 2018 / 225961 and further C-terminal amidation reaction was performed.
[0915] Peptides of the invention are prepared via analogous procedures, for example by substituting different amino acid building block reagents at the desired steps.
[0916] Exemplary preparation of YDE-093
[0917] To prepare the C-terminal amidated peptide YDE-093, the synthetic procedure was carried out as depicted in Scheme A below. The Fmoc-protected 10-mer peptide (Fmoc-Hyp-H-Gly-Gln-Leu-Gly-Ala-Leu-Gly-Pro-Lys(Dde)-OH) was prepared according to the p...
Embodiment 3
[0949] Example 3: Evaluation of the eye protection effect of YDE derivatives on dry eye syndrome
[0950] Preparation of rats with dry eye syndrome
[0951] In order to evaluate the eye protection effect of YDE-001 to YDE-028 prepared in Example 1 on dry eye syndrome, a total of 320 Sprague-Dawley-type (Sprague-Dawley-type) male rats (OrientBio, Seungnam, Korea) for 7 days. Thereafter, dry eye syndrome was induced in 264 test rats by extraorbital lacrimal gland excision (hereinafter referred to as ELGE). Eight test rats without ocular abnormalities were sham-operated as a control group.
[0952] By inhalation of 2% to 3% isoflurane (Hana Pharm.Co., Hwasung, Korea), 70% N 2 O and 28.6% O 2 Rats were generally anesthetized using a rodent anesthesia machine (Surgivet, Waukesha, Wis., USA) and a respirator (model 687, Harvard Apparatus, Cambridge, UK). Thereafter, the extraorbital lacrimal gland in the subcutaneous region above the masseter muscle and below the optic nerve wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com