Synthesis method of dapoxetine and dapoxetine hydrochloride
A synthetic method, dapoxetine technology, applied in the field of organic drug synthesis, can solve the problems of high price, cumbersome reaction route, high production cost, etc., and achieve the effect of low cost, easy availability of raw materials, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
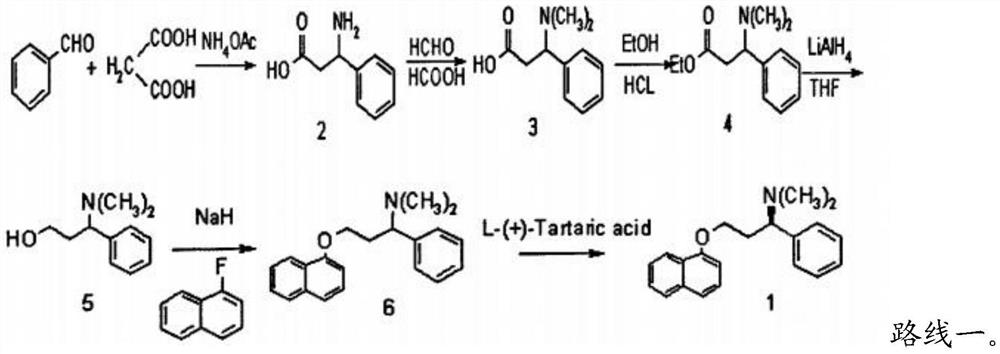
[0037] The invention provides a kind of synthetic method of dapoxetine, comprising the following steps:
[0038] (1) 1-naphthol, paraformaldehyde, hydrogen bromide and the first organic solvent are mixed, carry out bromomethylation reaction, obtain 1-naphthol bromomethyl ether;
[0039] (2) Under a protective atmosphere, the 1-naphthol bromomethyl ether, magnesium, an initiator and a second organic solvent are mixed to carry out a substitution reaction to obtain a 1-naphthol bromomethyl ether Grignard reagent solution;
[0040] (3) The 1-naphthol bromomethyl ether Grignard reagent solution, R-styrene oxide and the third organic solvent are mixed to carry out a Grignard reaction to obtain R-3-(1-naphthyloxy)- 1-phenylpropanol; R in the R-epoxy styrene includes tosyl, methanesulfonyl or m-nitrobenzenesulfonyl;
[0041] (4) Mix the R-3-(1-naphthyloxy)-1-phenylpropanol, the brominating reagent and the fourth organic solvent, and carry out the bromination reaction to obtain S-1-br...
Embodiment 1
[0089] (1) 1-naphthol bromomethyl ether
[0090] Add 400g of dichloromethane, 144.2g of 1-naphthol (1mol) and 36g of paraformaldehyde (1.2mol) into a 1000mL reaction flask, stir to form a suspension, feed dry hydrogen bromide gas, and control the temperature of the system not to exceed 30 °C until the solids are completely dissolved, transfer the reaction solution into a separatory funnel, add anhydrous sodium sulfate to the obtained lower organic phase to dry, filter, and distill the resulting filtrate to constant weight under reduced pressure to obtain 1-naphthol bromomethyl ether (Pale yellow oily liquid).
[0091] (2) R-3-(1-naphthyloxy)-1-phenylpropanol
[0092] Mix 0.5mol 1-naphthol bromide with 300mL tetrahydrofuran to obtain a 1-naphthol bromide solution; under nitrogen protection, add 150mL tetrahydrofuran, 14.6g magnesium strips (0.6mol) and 0.1g iodine and mix well , add 20mL of 1-naphthol bromide methyl ether solution dropwise, and slowly heat to reflux. When the...
Embodiment 2
[0101] (1) 1-naphthol bromomethyl ether
[0102] Add 400g of dichloromethane, 144.2g of 1-naphthol (1mol) and 36g of paraformaldehyde (1.2mol) into a 1000mL reaction flask, stir to form a suspension, feed dry hydrogen bromide gas, and control the temperature of the system not to exceed 30 °C until the solids are completely dissolved, transfer the reaction solution into a separatory funnel, add anhydrous sodium sulfate to the obtained lower organic phase to dry, filter, and distill the resulting filtrate to constant weight under reduced pressure to obtain 1-naphthol bromomethyl ether (Pale yellow oily liquid).
[0103] (2) R-3-(1-naphthyloxy)-1-phenylpropanol
[0104] Mix 0.5mol 1-naphthol bromide methyl ether with 300mL methyl tert-butyl ether to obtain 1-naphthol bromide methyl ether solution; under nitrogen protection, add 150mL methyl tert-butyl ether, 18.2g magnesium bar (0.75mol) and 1mL ethyl bromide were mixed evenly, and 15mL of 1-naphthol bromomethyl ether solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com