Method for detecting genotoxic impurities in safinamide mesylate
A technology of saphiramide mesylate and genotoxicity, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems that the detection sensitivity cannot meet the content requirements and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The embodiment of the present invention provides a kind of detection method of genotoxic impurity in safinamide mesylate, described detection method comprises the following steps:
[0050] Step 1, preparing the test solution, reference solution, blank solution and mixed solution;
[0051] The preparation method of the above-mentioned reference substance solution is: take (S)-4-(((1-amino-1-oxypropane-2-methyl)amino)methyl)benzene mesylate as reference substance, and methanol as solvent to obtain a concentration of 305.45ng / mL stock solution, and then dilute the above stock solution to obtain a concentration of 15ng / mL reference substance solution.
[0052] The preparation method of above-mentioned need testing solution is: get need testing 10mg and place in 10mL volumetric flask, adopt methanol to dissolve and dilute value scale, obtain the need testing solution that concentration is 1mg / mL.
[0053] The blank solution is methanol;
[0054] The preparation method of t...
Embodiment 2
[0064] Embodiment 2 detection limit and quantitative limit
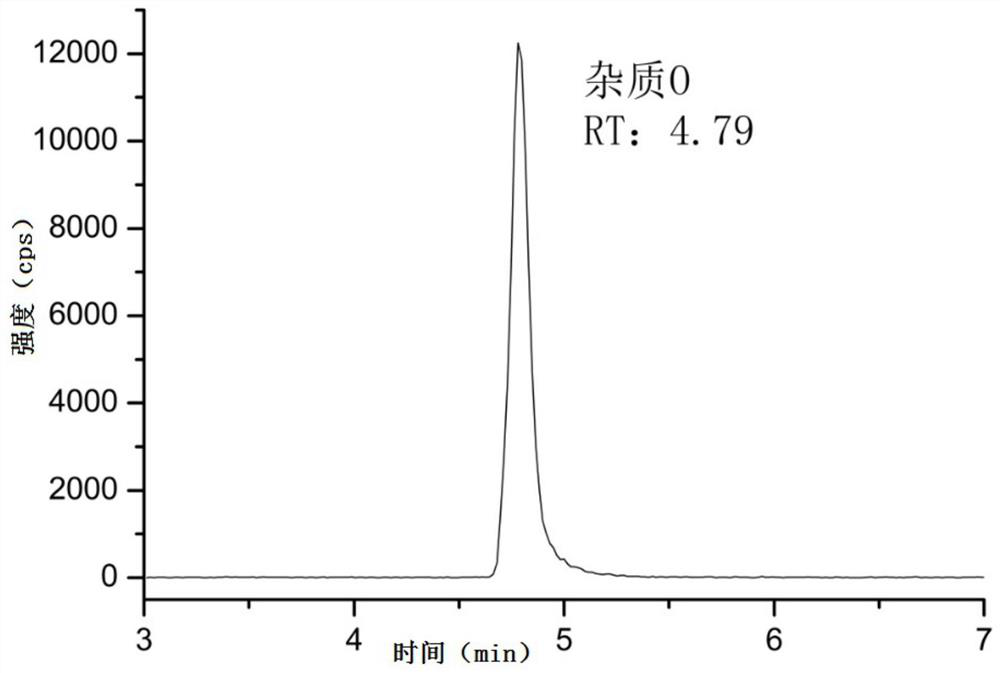
[0065] Detection limit: the concentration of 15ng / mL reference substance solution prepared in Example 1 is quantitatively diluted step by step with methanol, and then detected by liquid chromatography-mass spectrometry, the specific conditions of liquid chromatography and mass spectrometry are as described in Example 1 , record the spectrogram, and obtain the detection limit according to the signal-to-noise ratio not lower than 3:1, and the results are shown in Table 1.
[0066] Limit of quantification: the concentration prepared in Example 1 is that the 15ng / mL reference substance solution is quantitatively diluted step by step with methanol, and then the liquid chromatography-mass spectrometry method is used to detect the limit of quantification solution. The specific conditions of liquid chromatography and mass spectrometry are as follows: As described in Example 1, record the spectrogram, and obtain the quantific...
Embodiment 3
[0072] Example 3 Linearity
[0073] The concentration of 305.45ng / mL reference substance stock solution prepared in Example 1 was diluted with methanol to obtain concentrations of 1.53ng / mL, 3.05ng / mL, 9.16ng / mL, 15.27ng / mL, 21.38ng / mL, 30.54ng / mL linear solution.
[0074] Adopt liquid chromatography-mass spectrometry to detect linear solution, the specific conditions of liquid chromatography and mass spectrometry are as described in Example 1, record spectrogram information, then take the concentration (ng / mL) of reference substance impurity O as abscissa , take the peak area as the ordinate, draw the standard curve, and calculate the regression equation, the results are shown in Table 3, and the linear graph is shown in Figure 5 . from Figure 5 It can be seen that the impurity O has a good linear relationship within the concentration range of 1.53-30.54 ng / mL.
[0075] Table 3 Impurity O linearity test results
[0076]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com