Imbibition controlled-release antibacterial peptide hydrogel double-layer dressing as well as preparation method and application thereof
A gel layer and antimicrobial peptide technology, applied in the field of inhalation and controlled release antimicrobial peptide hydrogel double-layer dressing and its preparation, can solve the problems of ineffective wound healing, susceptibility to infection, non-biodegradability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation and characterization of hydrogel double-layer dressing with imbibition and controlled release of antimicrobial peptides
[0036] (1) Preparation method of inhalation and controlled-release antimicrobial peptide hydrogel double-layer dressing
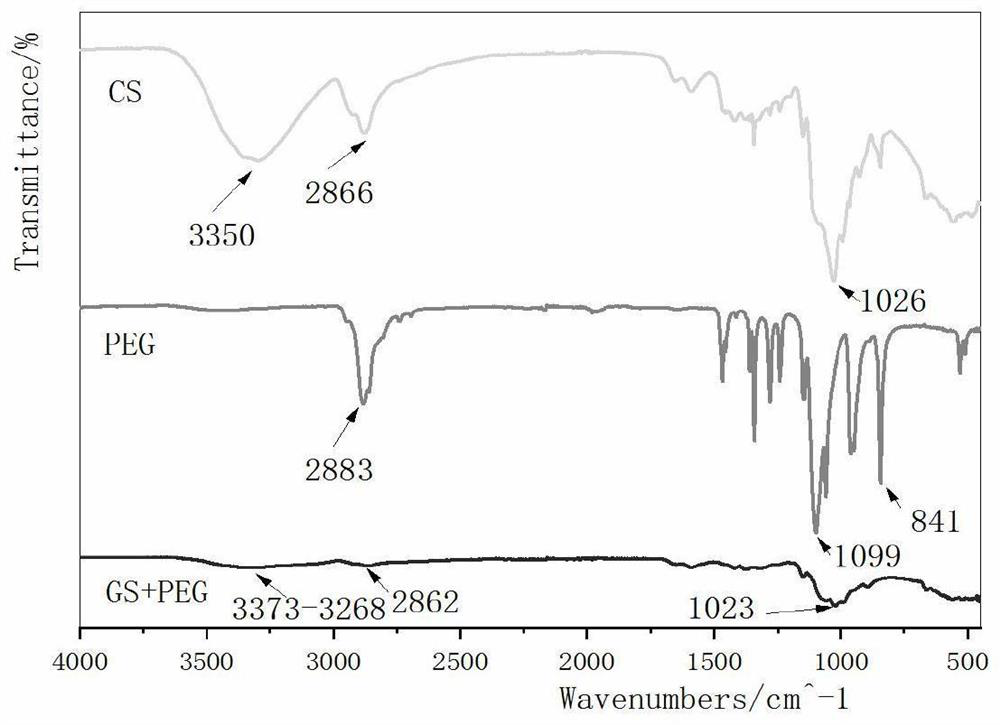
[0037] A. Dissolve CS (chitosan) in glacial acetic acid, stir overnight at 300r / min, filter out the undissolved CS with four layers of sterile gauze, add polyethylene glycol and glycerin to the chitosan solution, stir at room temperature for 2h, Centrifuge at 1500r / min for 10min, then pour the mixture into a petri dish, freeze overnight at -80°C, and freeze-dry in vacuum to obtain the lower layer of imbibition gel; the mass fractions of CS, PEG4000, and glycerol are 3%, 0.6%, and 1%;
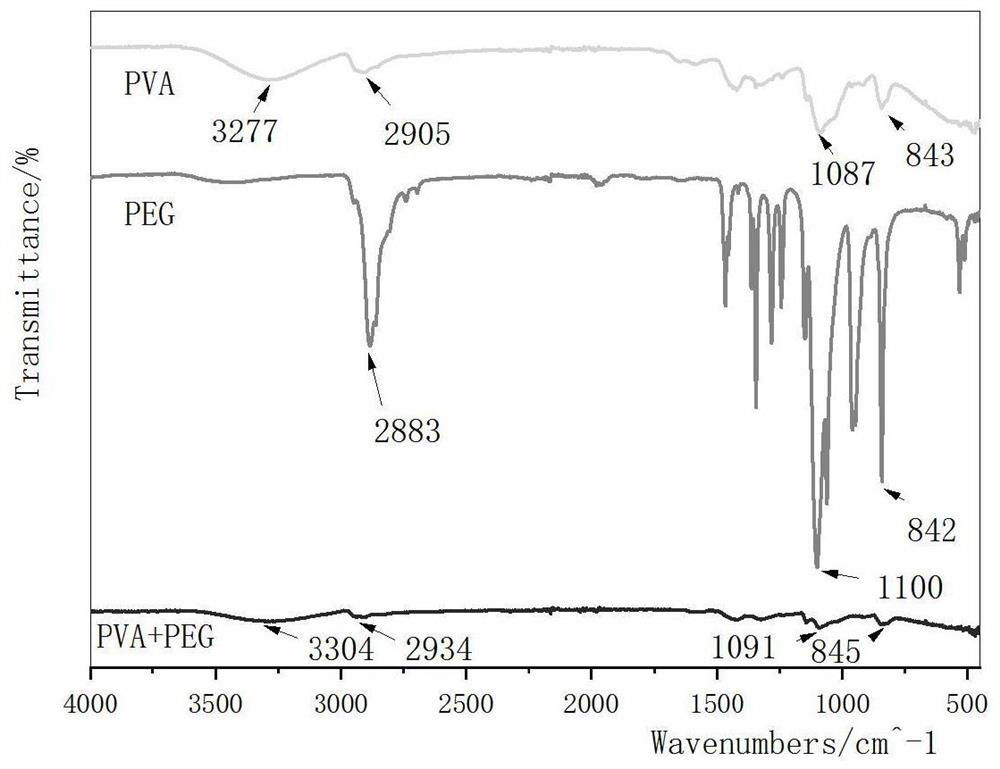
[0038] B. Mix polyvinyl alcohol and polyethylene glycol, add proSP-B antimicrobial peptide powder, so that the final concentration of proSP-B antimicrobial peptide is 1000 μg / mL, mix well, freeze overnight at -80°C, and freeze-thaw once...
Embodiment 2
[0046] Bacteriostatic activity of osmotic controlled-release antibacterial peptide hydrogel double-layer dressing against MRSA
[0047] The imbibition gel layer and proSP-B antimicrobial peptide controlled-release hydrogel were prepared according to the method in Example 1, and after being sterilized at high temperature, they were dried in an oven and sterilized by ultraviolet light for more than 2 hours. The two-layer gel was physically bonded to prepare a double-layer dressing of hydrogel for inhalation and controlled release of antimicrobial peptides. The imbibition gel layer was infiltrated with a sterile PBS solution with a pH of 7.2, imitating the pH value of the wound infection, and then the infiltration controlled-release antimicrobial peptide hydrogel double-layer dressing was covered on the plate coated with MRSA, and the temperature was maintained at 37 °C. After culturing for 18 hours, measure the size of the inhibition zone with a ruler, and perform statistical an...
Embodiment 3
[0050] Evaluation of hemolytic property of double-layer dressing of antimicrobial peptide hydrogel with inhalation and controlled release
[0051] 1. Sample preparation:
[0052] Experimental group: 0.05g freeze-dried dressing / 10mL normal saline
[0053] Positive control group: 10mL distilled water
[0054] Negative control group: 10mL normal saline
[0055] 2. Fresh anticoagulant blood: Potassium oxalate anticoagulant rabbit blood
[0056] 3. Preparation of diluted anticoagulant blood: 4mL anticoagulant blood + 0.2mL potassium oxalate solution (20g / L) + 5mL normal saline, fully shake the three to obtain diluted anticoagulant blood.
[0057] 4. Experimental procedure: Take 6 clean centrifuge tubes (15mL size), and set up 3 parallel groups in each group, respectively numbered as positive control, negative control, suction layer, release layer, double layer (without proSP-B ), double layer (containing 1000μg / mL proSP-B). 0.05g of dressings with different numbers were added ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com