Marker for viral pneumonia and application of marker
A viral pneumonia, marker technology, applied in antiviral agents, medical preparations containing active ingredients, disease diagnosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preliminary clinical trial of celecoxib
[0044] In this example, on the basis of conventional treatment following the guidelines, in cooperation with the Guangzhou Eighth People's Hospital, a clinical study of the type II cyclooxygenase inhibitor celecoxib in the treatment of new coronary pneumonia was carried out. Cloth for clinical research, oral administration for 10-14 days, twice a day, 200 mg each time, the specific cases are as follows:
[0045] The first batch of 8 cases were enrolled in the test group (4 cases of severe disease, 4 cases of common type), and 7 cases of the control group (7 cases of common type). Follow-up evaluations were conducted on the 4th and 10th days of treatment and found that the condition had improved. All of them were improved, and there was no serious condition; the condition of the control group was aggravated, unchanged and improved, respectively 42%, 14% and 42%. The curative effect of the experimental group was signifi...
Embodiment 2
[0046] Embodiment 2 celecoxib expanded clinical trial
[0047] In this example, in order to further verify the clinical research of the type II cyclooxygenase inhibitor celecoxib in the treatment of new coronary pneumonia, further increase the number of enrolled groups, the specific cases are as follows:
[0048] As of February 23, 2020, 31 cases (6 cases of severe disease and 25 cases of common type) were formally enrolled in the trial group. The specific medication and results are shown in Table 1 below:
[0049] Table 1
[0050]
[0051] It can be seen from Table 1 that among all the 38 cases including the test group and the control group, 84.2% (32 / 38) of the patients were admitted with mild disease (mild or common CT imaging manifestations), and 15.7% ( 6 / 38) patients were seriously ill (severe) when they were admitted to the hospital, after treatment (1) 31 / 31 cases improved (100% improvement rate); (2) 31 / 31 cases of virus turned negative (negative rate 100%) ; (3)...
Embodiment 3
[0052] Clinical diagnosis of embodiment 3 PGE2
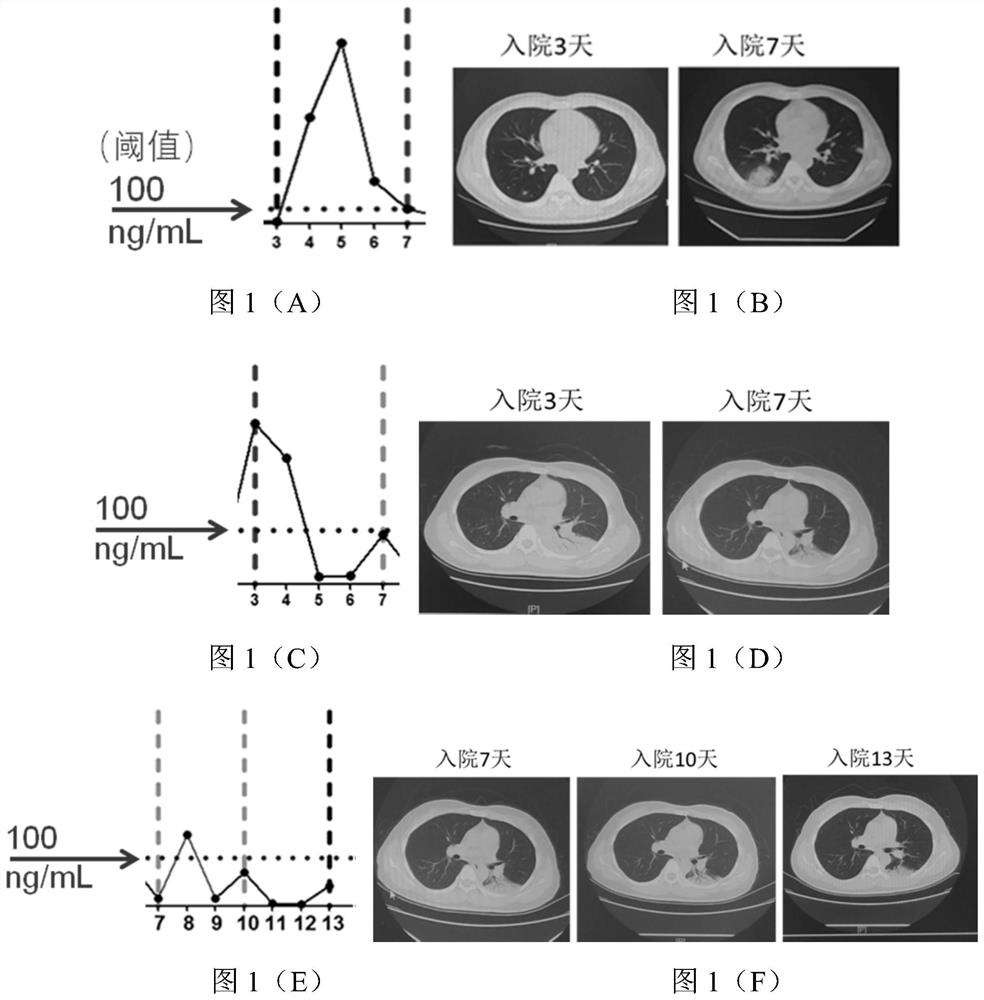
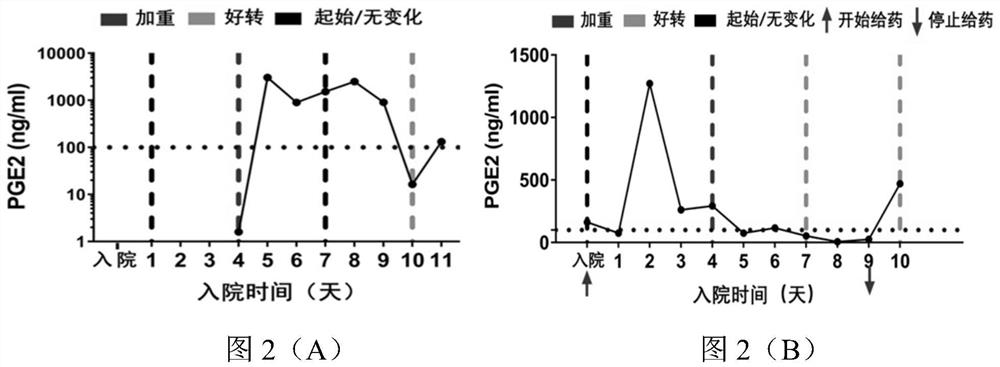
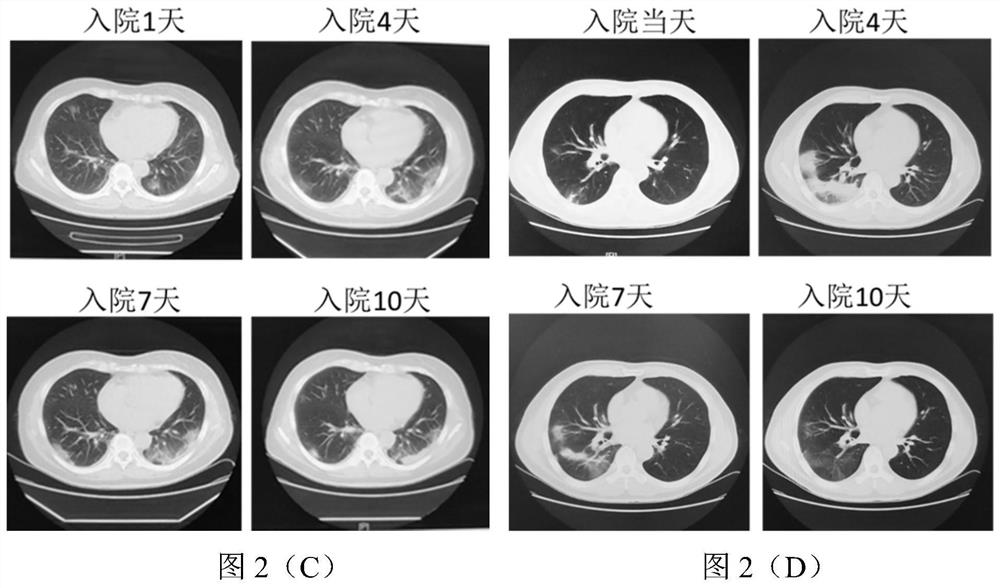
[0053] In this example, in order to further verify the clinical research of the type II cyclooxygenase inhibitor celecoxib in the treatment of new coronary pneumonia, and further analyze the correlation between PGE2 and changes in the condition of new coronary pneumonia, this example tracks and detects patients with new coronary pneumonia every day The level of PGE2 in the urine, and compared with the CT results, the results are as follows figure 1 shown.
[0054] From figure 1 It can be seen that there are three patterns of PGE2 level changes between two adjacent CT detections: 1) figure 1 In (A) there is a climbing peak exceeding 1000ng / ml; 2) figure 1 (C) falls from a peak value exceeding 1000 ng / ml; 3) figure 1 In (E), the small amplitude vibration is lower than the baseline of 100ng / ml; corresponding to the analysis of two adjacent CT results, there are also three corresponding disease regressions, such as figure 1 (B)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com