Antiviral solution preparation for aerosol inhalation and preparation method thereof
A technology of atomization inhalation, antiviral oral liquid, applied in the field of preparation, can solve the problems of slow onset, large toxic side effects, large dosage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
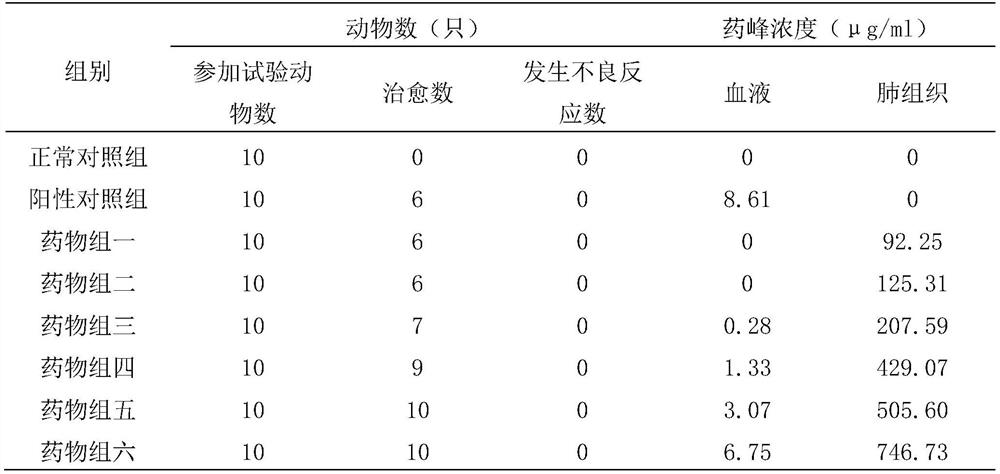
Image
Examples
Embodiment 1
[0037] (1) Preparation of active ingredients:
[0038] 12.87 parts of isatidis, 4.65 parts of forsythia, 5.72 parts of gypsum, 2.5 parts of anemarrhena, 3.22 parts of rehmannia glutinosa, 2.50 parts of calamus, 2.50 parts of turmeric, 2.86 parts of patchouli, 6.08 parts of reed root
[0039] Weigh Jiuwei medicine according to the above weight, add water and decoct twice, collect volatile oil for 3 hours for the first time, and clathrate with hydroxypropyl beta cyclodextrin, or collect volatile oil and volatile oil emulsion at the same time for 1.5 hours for the first time Turbid liquid; for the second time, filter for 1 hour and 20 minutes, combine the filtrates, concentrate to an appropriate amount, add more than 85% ethanol to make the alcohol content 70%, let stand, filter, recover ethanol from the filtrate and concentrate to an appropriate amount, add The inclusion compound of volatile oil or adding volatile oil and volatile oil emulsion to obtain the active ingredient of ...
Embodiment 2
[0045] (1) Preparation of active ingredient: same as Example 1.
[0046] (2) Preparation of antiviral atomized inhalation solution:
[0047]
[0048] Weigh the active ingredient according to the prescription amount, add an appropriate amount of water for injection, and stir evenly to obtain solution 1; take sodium chloride, add appropriate amount of water for injection, stir to dissolve, and obtain solution 2; combine solution 1 and solution 2, and stir evenly to obtain solution 2. Solution 3: Take another appropriate amount of disodium hydrogen phosphate, add appropriate amount of water for injection to prepare a 0.4mol / L solution, stir slowly and add it to solution 3, adjust the pH value to 4.0-7.0, add 1000ml of water for injection, fill and seal, Instantly.
Embodiment 3
[0050] (1) Preparation of active ingredient: same as Example 1.
[0051] (2) Preparation of antiviral atomized inhalation solution:
[0052]
[0053]
[0054]Weigh the active ingredient according to the prescription amount, add an appropriate amount of water for injection, and stir evenly to obtain solution 1; take magnesium chloride and citric acid, add appropriate amount of water for injection, stir to dissolve, and obtain solution 2; combine solution 1 and solution 2, stir evenly, Obtain solution 3; take another appropriate amount of disodium hydrogen phosphate, add appropriate amount of water for injection to prepare a 0.2mol / L solution, stir slowly and add it to solution 3, adjust the pH value to 4.0-6.0, add 1000ml of water for injection, fill and seal , that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com