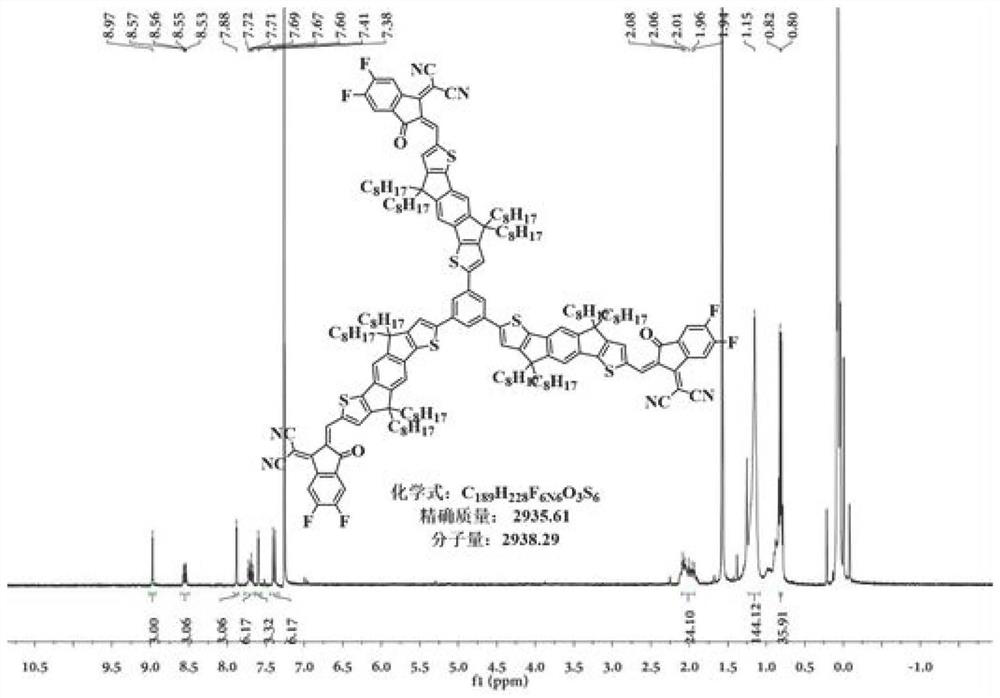
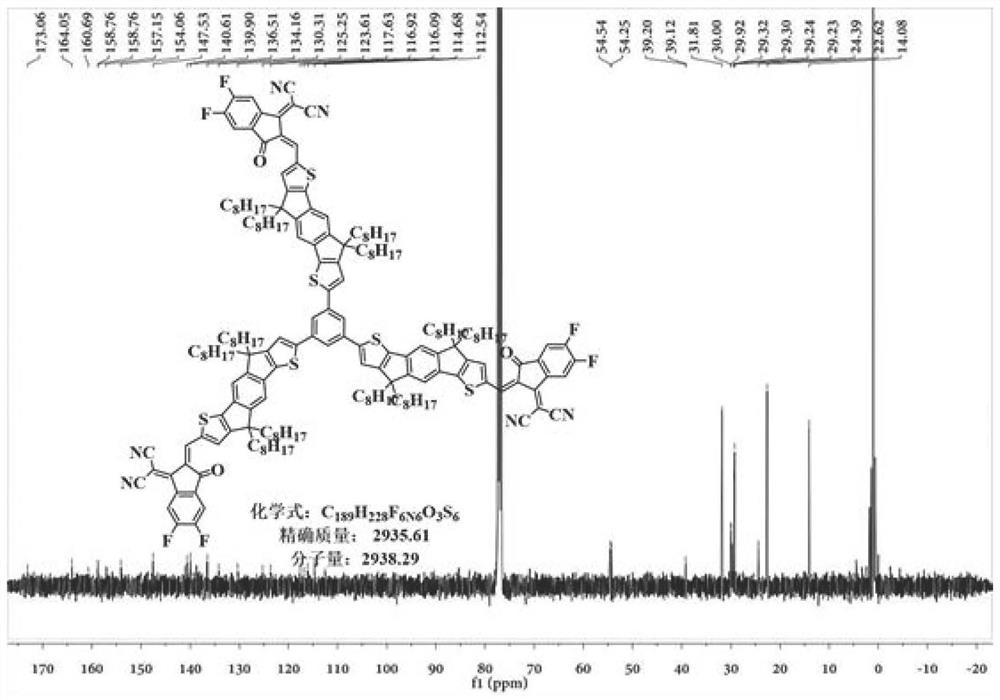
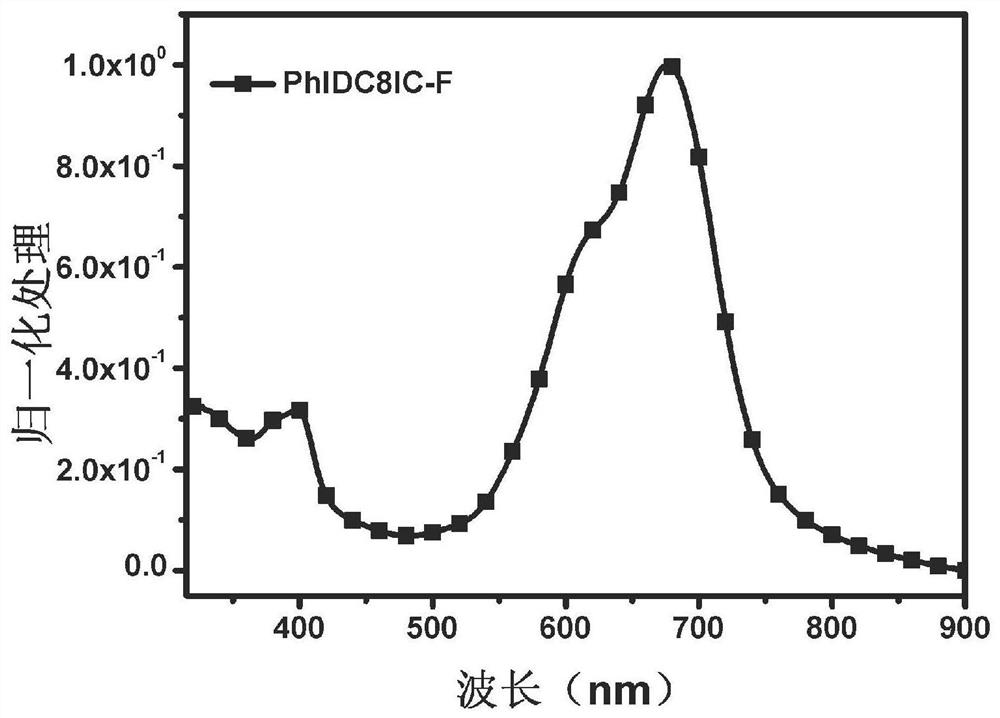
Non-fullerene electron acceptor material and preparation method and application thereof
An electron acceptor material, non-fullerene technology, applied in electric solid state devices, chemical instruments and methods, semiconductor/solid state device manufacturing, etc., can solve the problems of poor material film stability and limited isotropic charge transport, etc. Achieve the effect of mature synthesis process, excellent solubility and film formation, and excellent molar absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] For the preparation of compound S-1, see Figure 5 ;
[0038] Specific steps are as follows:
[0039] Step Ⅰ: Take 4,4,9,9-tetraoctyl-4,9-dihydro-s-indan[1,2-b:5,6-b']dithiophene (a) (996.0mg , 1.394mmol), N-N dimethylformamide (b) (157.9mg, 1.812mmol) and phosphorus oxychloride (256.5mg, 1.673mmol) in the reaction flask, then add 20mL 1,2-dichloroethane, React at 100°C for 24h. The obtained mother liquor was washed with water, extracted with dichloromethane, dried with anhydrous sodium sulfate, then evaporated the excess solvent by vacuum distillation, purified by silica gel column chromatography, and finally obtained solid c (403.5 mg, 40.0%) after spin-drying.
[0040] Step II: Compound c (389.4mg, 0.524mmol) was dissolved in 10mL of dichloromethane in a reaction flask, protected from light, and stirred in an ice-water bath. Take N-bromosuccinimide (d) (121.2mg, 0.681mmol) and dissolve it in 10mLDMF, and drop it into two-necked reaction flasks drop by drop, react...
Embodiment 2
[0045] The preparation step of S-2 compound sees Figure 6 .
[0046] Formula S-2 was prepared in a similar manner to compound S-1, except that 1,3,5-benzenetriboronic acid tripinacol ester (H-1) was replaced by H-2. The yield of the first step is 41.8%, the yield of the second step is 91.7%, the yield of the third step is 38.6%, and the yield of the fourth step is 90.4%.
[0047] Compound S-2 product MS (m / z): 3113.68; Elemental analysis (C 203 h 236 f 6 N 6 o 3 S 6 ): C, 78.29; H, 7.64; F, 3.66; N, 2.70; O, 1.54; S, 6.18.
Embodiment 3
[0049] The preparation process of S-7 compound is shown in Figure 7 :
[0050] Formula S-7 was prepared in a similar manner to compound S-1, except that H-7 was used in place of 1,3,5-benzenetriboronic acid tripinacol ester (H-1). The yield of the first step is 40.6%, the yield of the second step is 90.3%, the yield of the third step is 37.5%, and the yield of the fourth step is 91.4%.
[0051] Compound S-7 product MS (m / z): 3243.68; Elemental analysis (C 207 h 234 f 6 N 12 o 3 S 6 ): C, 76.63; H, 7.27; F, 3.51; N, 5.18; O, 1.48; S, 5.93.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar extinction coefficient | aaaaa | aaaaa |
| Electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com