Organic electron acceptor material and preparation method and application thereof
A technology for acceptor materials and organic electronics, which is applied in the field of organic electron acceptor materials and their preparation, and can solve the problems that star-shaped non-fullerene electron acceptor materials have not been optimized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
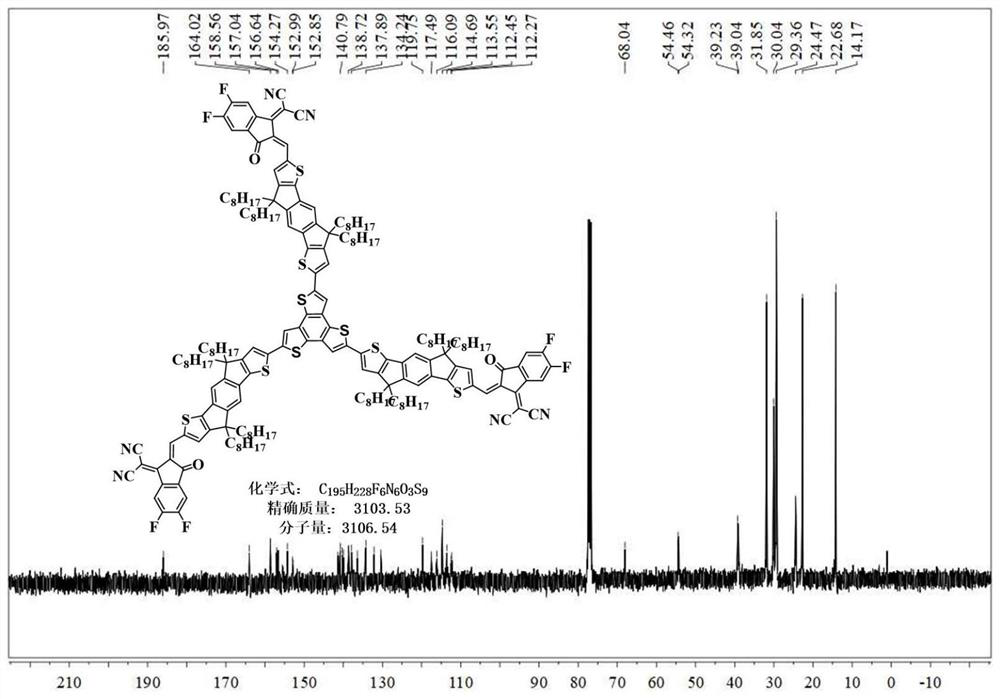
[0037] The preparation process of S-3 compound is shown in Figure 5 .
[0038] Specific steps are as follows:
[0039] The first step: take 4,4,9,9-tetraoctyl-4,9-dihydro-s-indan[1,2-b:5,6-b']dithiophene (C-3)( 996.0mg, 1.394mmol), N-N dimethylformamide (a) (157.9mg, 1.812mmol) and phosphorus oxychloride (b) (256.5mg, 1.673mmol) in the reaction flask, then add 20mL 1,2- Dichloroethane was reacted at 100°C for 24h. The obtained mother liquor was washed with water, extracted with dichloromethane, dried with anhydrous sodium sulfate, then evaporated the excess solvent by distillation under reduced pressure, purified by silica gel column chromatography, and finally spin-dried to obtain c (403.5 mg, 40.0%).
[0040] Step II: Dissolve compound c (389.4mg, 0.524mmol) in 10mL of dichloromethane in a reaction flask, and put it in an ice-water bath to stir in the dark. Take N-bromosuccinimide (d) (121.2mg, 0.681mmol) and dissolve it in 10mLDMF, and drop it into two-necked reaction ...
Embodiment 2
[0045] For the preparation process of S-10 compound see Image 6 .
[0046] Formula S-10 was prepared in a similar manner to compound S-3, except that C-10 was used to replace 4,4,9,9-tetraoctyl-4,9-dihydro-s-indan[1,2 -b:5,6-b']dithiophene (C-3). The yield of the first step is 41.1%, the yield of the second step is 89.8%, the yield of the third step is 39.5%, and the yield of the fourth step is 85.3%.
[0047] Compound S-10 product MS (m / z): 2960.09; Elemental analysis (C 171 h 180 f 6 N 6 o 15 S 9 ): C, 69.34; H, 6.13; F, 3.85; N, 2.84; O, 8.10; S, 9.74.
Embodiment 3
[0049] The preparation process of S-18 compound is shown in Figure 7 .
[0050] Formula S-18 was prepared in a similar manner to compound S-3, except that C-18 was used to replace 4,4,9,9-tetraoctyl-4,9-dihydro-s-indan[1,2 -b:5,6-b']dithiophene (C-3). The yield of the first step is 41.7%, the yield of the second step is 88.9%, the yield of the third step is 39.7%, and the yield of the fourth step is 86.2%.
[0051] Compound S-18 product MS (m / z): 3801.60; Elemental analysis (C 219 h 252 f 6 N 6 o 27 S 9 ): C, 69.17; H, 6.68; F, 3.00; N, 2.21; O, 11.36; S, 7.59.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com