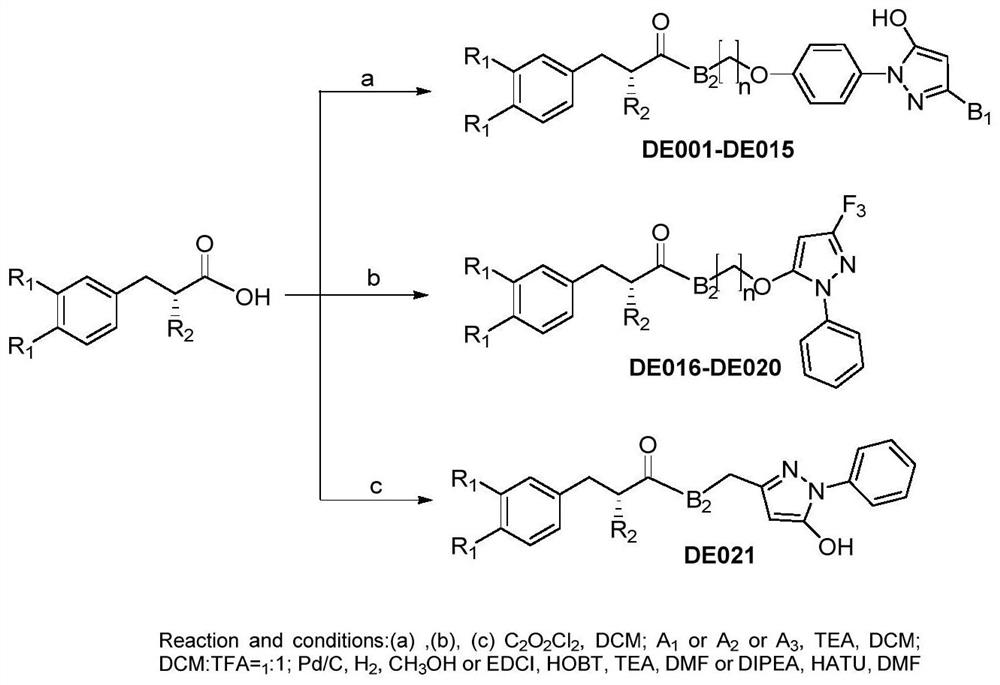
Danshensu derivativeS as well as preparation method and medical application thereof
A Danshensu derivative, Danshensu technology, applied in the direction of pharmaceutical formulations, drug combinations, bulk chemical production, etc., can solve the problems of limited application, strong hydrophilicity, unstable danshensu phenolic hydroxyl structure, etc., and achieve an improved balance Coordination ability, increasing drug stability, and reducing the effect of cerebral infarction volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
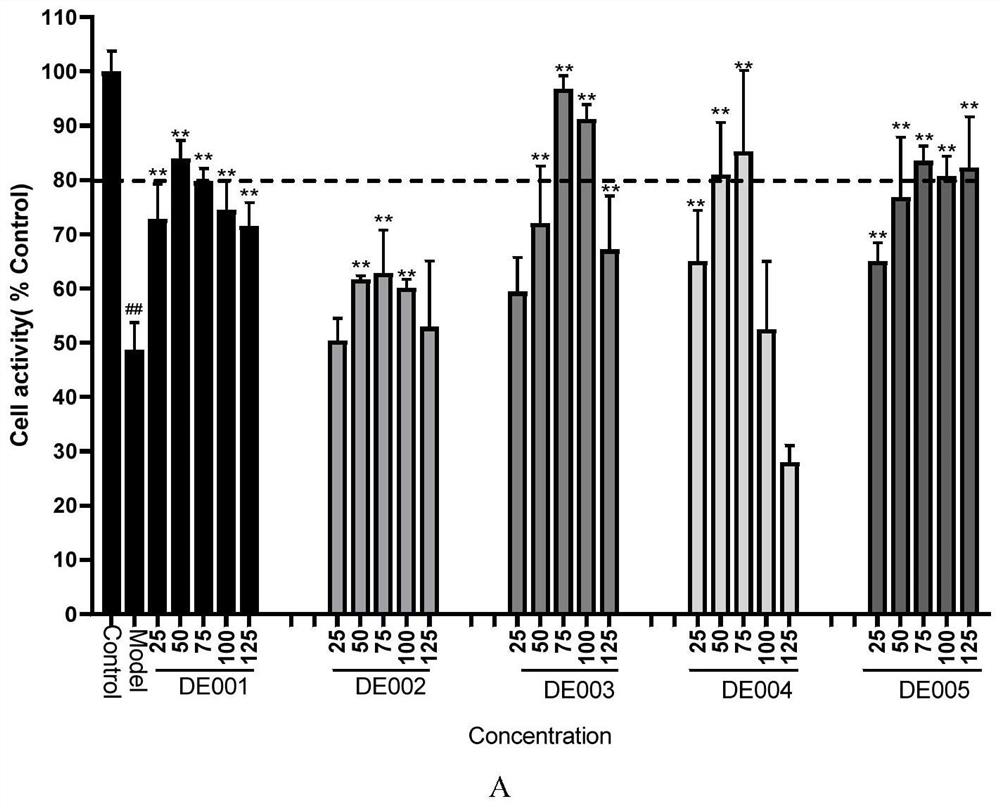
[0077] The synthesis of embodiment 1 compound DE001-DE005
[0078] The synthetic route is as follows:
[0079]
[0080] The specific synthetic method comprises the following 14 steps of reaction:
[0081] [1] Take a 250ml reaction bottle, dissolve danshensu sodium (10g, 45mmol) in DMF (200ml), magnetically stir to dissolve, add sodium bicarbonate (7.6g, 90mmol) to react at room temperature, monitor the reaction by TLC, after 6h the product point The concentration does not change anymore, and the reaction ends. Pour the reaction liquid into 300mL ice water, extract 2-3 times with ethyl acetate, combine the organic phases and wash with saturated brine 2-3 times, dry over anhydrous sodium sulfate, and rotary evaporate to obtain a yellow oily crude product, which is directly used in the next step without purification. .
[0082]
[0083] [2] Weigh compound (R)-3-(3,4-dihydroxyphenyl)-2-hydroxypropionate benzyl ester (2) (1 equivalent) and dissolve it in DMF (200ml), under...
Embodiment 2
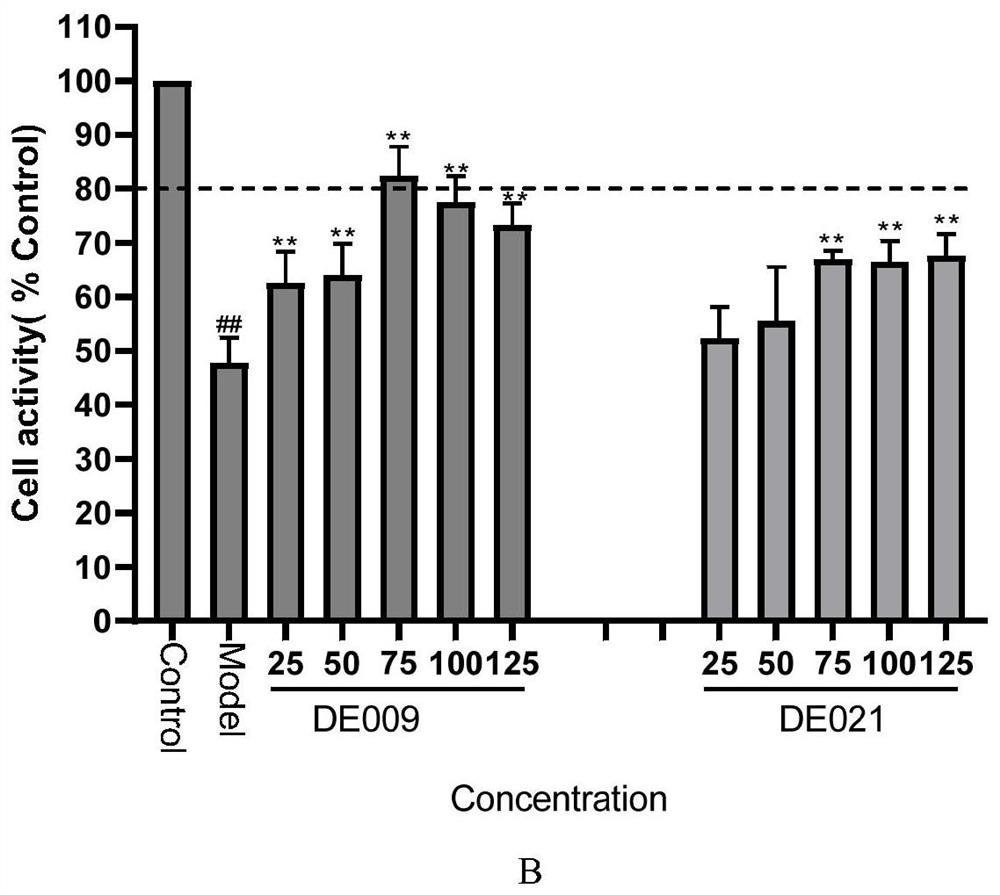
[0120] The synthesis of embodiment 2 compound DE009
[0121] The synthetic route is as follows:
[0122]
[0123] Concrete synthetic method, comprises following 5 steps reaction:
[0124] [1] Weigh compound 9 (1eq) into a reaction flask and add anhydrous methanol to dissolve it, add ethyl trifluoroacetoacetate (0.8eq), heat to 110-120°C for 4 hours, and evaporate the reaction system Dry and purify on a silica gel column (petroleum ether: ethyl acetate = 4:1-1:1) to obtain product 18 (45-63%).
[0125]
[0126] [2] Dissolve compound 1-(4-(benzyloxy)phenyl)-3-(trifluoromethyl)-1H-pyrazole-5-ol (18) (1 equivalent) in dichloromethane, Under magnetic stirring, Boc anhydride (1-1.5 equivalents), triethylamine (1.5-2 equivalents), and DMAP (0.1-0.3 equivalents) were sequentially added, reacted at room temperature, monitored by TLC, and the reaction was completed. The system was rotary evaporated to remove most of the solvents, the remaining reaction liquid was extracted 2-3 ...
Embodiment 3
[0138] The synthesis of embodiment 3 compound DE021
[0139] The synthetic route is as follows:
[0140]
[0141] Concrete synthetic method, comprises following 6 steps reaction:
[0142][1] Weigh benzyl alcohol (1eq) and dissolve it in 50ml THF, under magnetic stirring, cool to 0°C, dissolve the weighed NaH (1.2-1.5eq) in 20ml THF, and slowly drop it into the reaction flask , the reaction was continued for 30 min in an ice bath. The reaction system was cooled to 0° C. again, and ethyl 4-chloroacetoacetate (0.7-0.75 eq) was slowly added dropwise into the reaction system with an addition funnel. After reacting for 5 h, the end of the reaction was monitored by TLC. Rotate the system to remove the solvent, add a small amount of dilute hydrochloric acid and petroleum ether to extract 2-3 times, wash the organic phase with saturated brine 1-2 times, dry over anhydrous sodium sulfate, and rotate to evaporate. Purification by silica gel column chromatography (petroleum ether: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com