Conjugate, and preparation method and application thereof
A technology for conjugates and compounds, applied in the field of conjugates and their preparation, can solve the problems of small production scale, complicated operation, poor flexibility of nucleic acid conjugates, etc., and achieve good practicability, broad application prospects, and high specificity. The effect of recognition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0079] This preparation example is used to illustrate the preparation of azide-modified targeting ligands 6a-c
[0080] n=2, 5, 12.
[0081] synthetic route:
[0082]
[0083] Specific steps:
[0084]
[0085] (i) Polyethylene glycol 1a-c (100mmol, 1eq) was dissolved in dry THF (150mL), metal sodium (230mg, 10mmol, 0.1eq) was added thereto, and stirred at room temperature until the sodium was completely dissolved. Subsequently, tert-butyl acrylate (6.4 g, 50 mmol, 0.5 eq) was slowly added dropwise to the mixture, and reacted at room temperature for 12 hours. After the reaction was completed, 8 mL of 1M aqueous hydrochloric acid solution was added to quench the reaction. After stirring for 10 minutes, the reaction solution was poured into 200 mL of saturated brine, extracted 3 times with ethyl acetate, the organic phase was collected, washed with saturated brine, dried over anhydrous sodium sulfate, separated and purified by flash column chromatography (PE / EA=10 :1-...
preparation example 2
[0122] This preparation example is used to illustrate the synthesis of 3'-terminal propargyl-modified small nucleic acids
[0123] 1-O-propargyl-2-deoxy-D-furanose (X, i.e. propargyl monomer), as a general overhang modification, was ligated by using an ABI 394 DNA / RNA synthesizer (ABI, USA) to the 3'-end of small nucleic acids (oligonucleotides) (such as figure 1 shown).
[0124] The specific steps for the synthesis of 3'-terminal propargyl-modified small nucleic acids are as follows: First, the 1-O-propargyl-2-deoxy-D-furanose phosphoramidite monomer was synthesized from commercial Hoff chloride sugar body (Bioorg.Med.Chem., 2013, 5583–5588); then, after the obtained phosphoramidite monomer is connected to a solid phase carrier (CPG), the propargyl monomer X oligonucleotide is synthesized by the aforementioned automatic nucleic acid synthesizer The 3'-end of the acid; finally, remove the CPG and the protecting group to obtain different 3'-end propargyl-modified single-stran...
Embodiment 1
[0128] This example is used to illustrate the efficient synthesis of KUE-PEG-siRNA conjugates
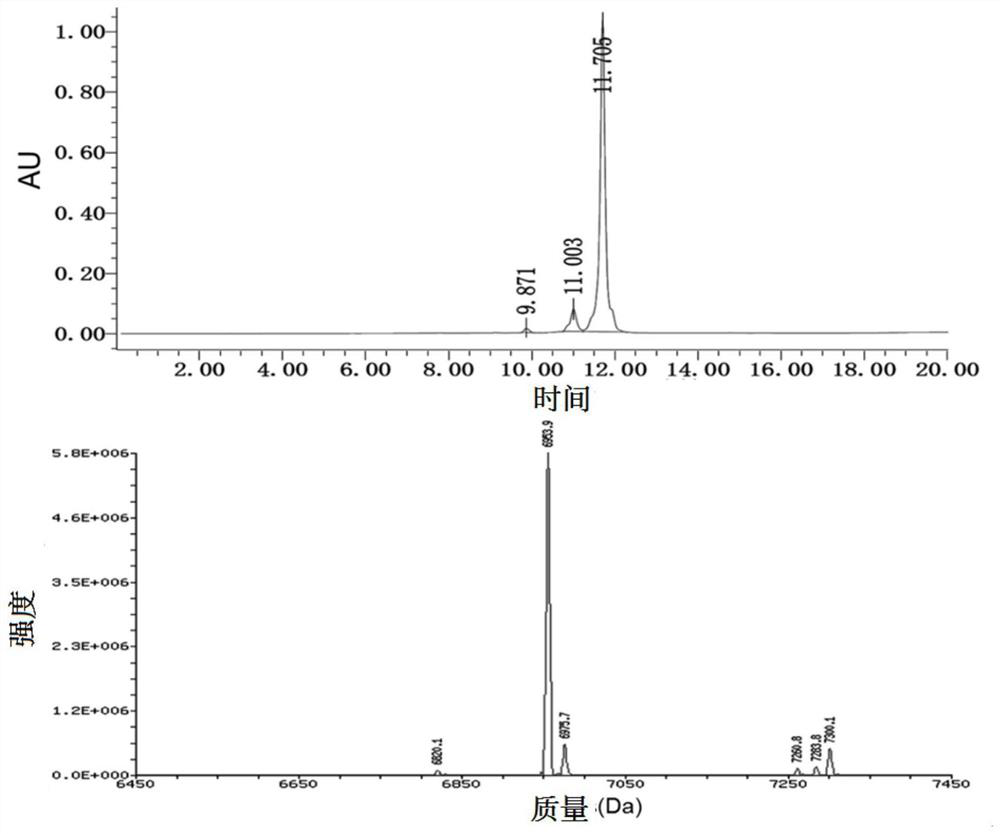
[0129] KUE-PEG 2 -Synthesis of siRNA conjugates: Add propargyl-modified small nucleic acid sequence (ON3) (10 nmol), azide-modified targeting ligand 6a (30 nmol), Cu(I)- TBTA (50nmol), so that the final concentration of RNA was maintained at about 100μM. Add DMF to the system (the volume accounts for 25% of the reaction solution volume). The reaction system was gently vortexed and mixed, placed in a metal reactor, shaken and reacted at 37°C and 900r / min for 3h, and the reaction results were monitored by 15% denaturing polyacrylamide gel electrophoresis. Finally, the product was separated and purified by an oligonucleotide extraction kit, the purity and concentration of the product were confirmed by a NanoDrop 2000 ultramicro spectrophotometer (Thermo, USA), and the final yield was 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com