Electrochemical analysis method constructed based on bovine serum albumin-ruthenium dioxide nanoparticles and used for detecting intracellular hydrogen peroxide
A technology for bovine serum albumin and electrochemical analysis, applied in the field of electrochemistry, can solve problems such as being easily affected by interferents, difficult to detect with high sensitivity, and time-consuming, and achieve fast detection speed, good reproducibility, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
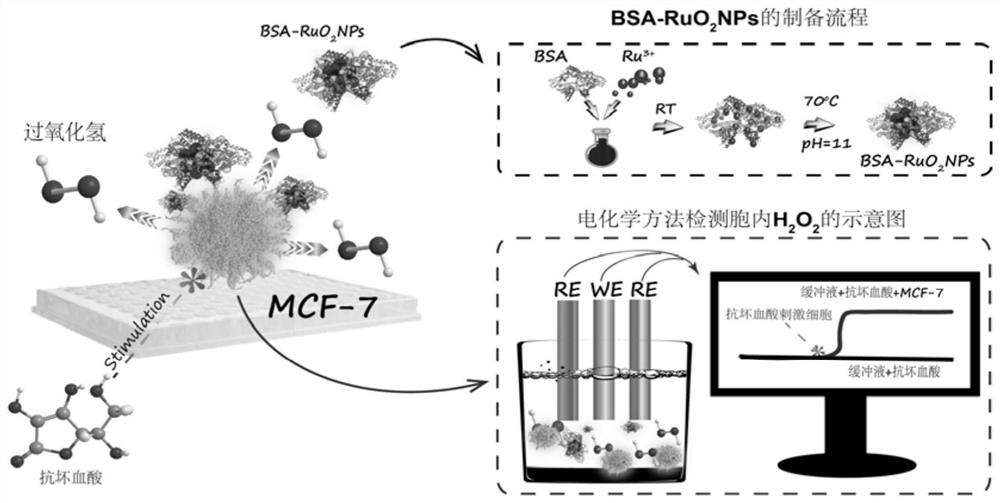
[0034] BSA-RuO 2 The preparation method of NPs was as follows: 1.6 mL of bovine serum albumin with a concentration of 18.75 mg / mL was mixed with 0.4 mL of ruthenium trichloride with a concentration of 75 mmol / L, stirred at 37 °C for 90 minutes to ensure uniform mixing, and then The pH of the above system was adjusted to 11 with sodium hydroxide, and the reaction was fully stirred at 70° C. for 2.5 hours. After the reaction, the solution was black. After the reaction, the solution was put into an ultrafiltration tube with a molecular weight cut-off of 3k, subjected to centrifugal ultrafiltration at 6000 r / min, and washed with water three times. After the volume was fixed to 2 mL, it was bovine serum albumin-ruthenium dioxide nanoparticles (2 mg / mL) required for the experiment, and stored at 4°C. figure 1 for BSA-RuO 2 Preparation process of NPs and detection of intracellular H by electrochemical method 2 o 2 schematic diagram.
example 2
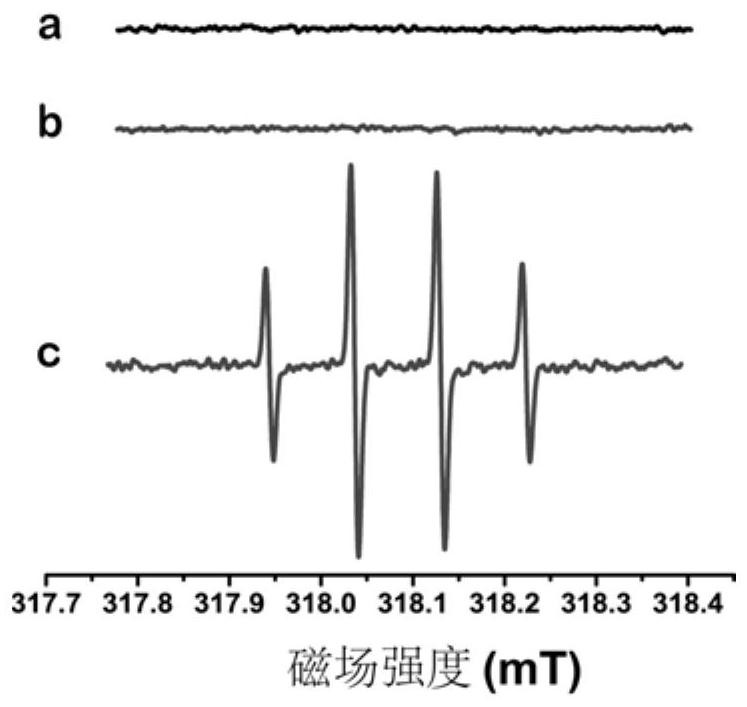
[0036] Free radical scavengers can successfully detect superoxide radicals and hydroxyl radicals generated in vivo or in vitro. 5,5-Dimethyl-1-pyrroline N-oxide is a commonly used hydroxyl radical scavenger, such as figure 2 As shown in the electron spin resonance spectrum, the BSA-RuO prepared in Example 1 2 NPs can catalyze H 2 o 2 Generate hydroxyl radicals to interact with 5, 5-dimethyl-1-pyrroline N-oxide, thus proving that BSA-RuO 2 NPs can efficiently catalyze H 2 o 2 .
example 3
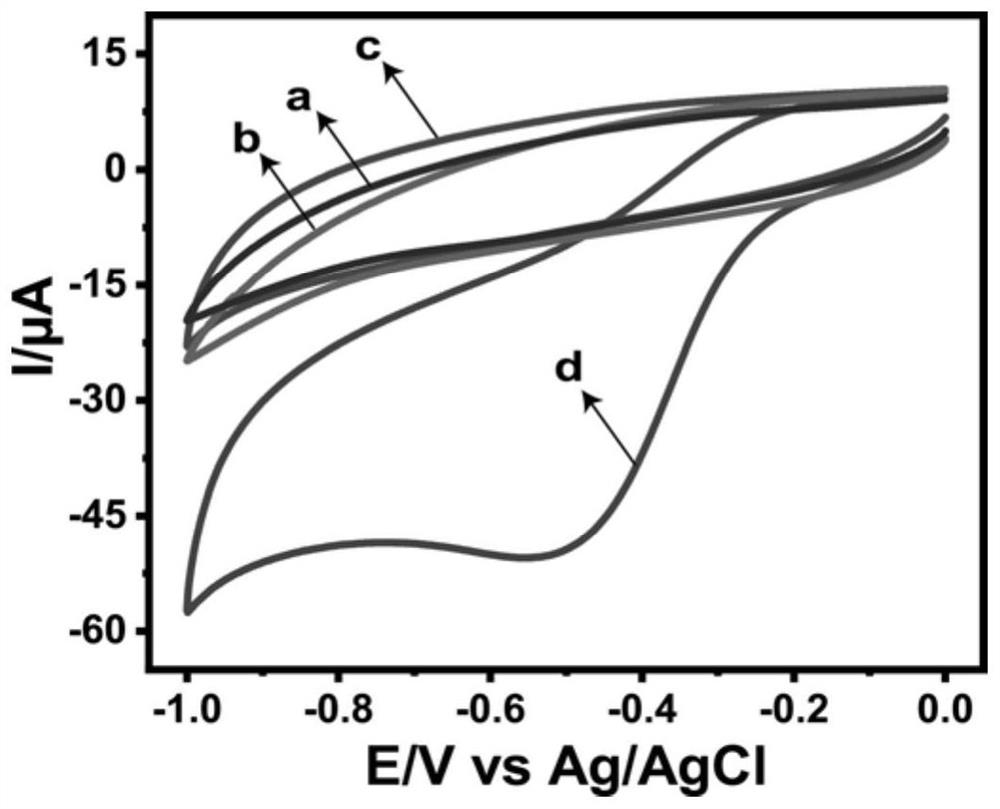
[0038] Utilize cyclic voltammetry to examine the BSA-RuO that example 1 makes 2 NPs / GCE versus H 2 o 2 electrochemical catalysis, such as image 3 As shown, from a to d are respectively unmodified GCE, H 2 o 2 + GCE, BSA-RuO 2 NPs / GCE and H 2 o 2 + BSA-RuO 2 Cyclic voltammetry curves of NPs / GCE at a scan rate of 50 mV / s and pH=7.0 (0.05 mol / L PBS). In the absence of H 2 o 2 case of GCE(a) and BSA-RuO 2 NPs / GCE (c) did not detect obvious redox peaks. Under bare GCE (b) condition for H 2 o 2 The response is negligible, indicating that unmodified BSA-RuO 2 GCE of NPs applied to electrochemical H 2 o 2 Detection performance is poor. As shown in (d), BSA-RuO 2 NPs / GCE versus H 2 o 2 There is an obvious catalytic effect, and an obvious reduction peak appears at about -0.5 V, with a peak current of 50.54 µA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com