Graphdiyne nano preparation, preparation method thereof and application of graphdiyne nano preparation in preparation of medicine for treating Parkinson's disease
A nano-preparation and graphyne technology, applied in the direction of nano-medicine, nano-carbon, drug combination, etc., can solve the problems of lack of treatment methods, achieve high drug loading capacity, improve brain delivery efficiency, and improve brain drug delivery efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
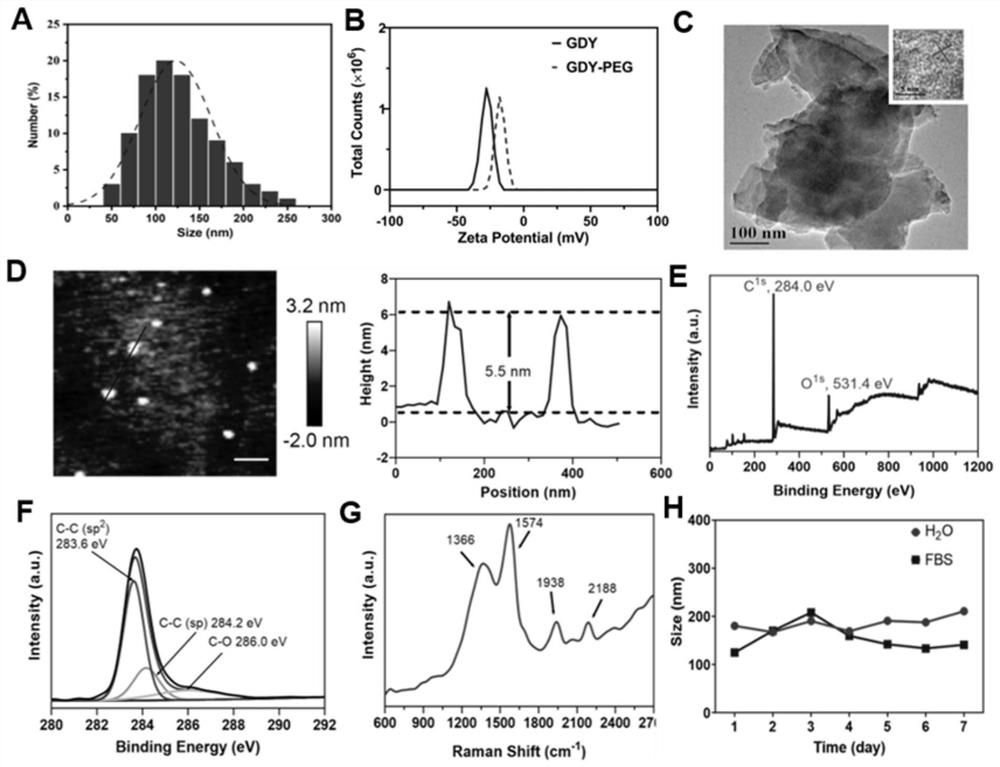
[0059] An embodiment of the present invention, this embodiment provides a preparation method of GDY and PEG-GDY, the preparation method is:
[0060] (1) graphyne preparation:
[0061] 43.6 mg of hexa[(trimethylsilyl)ethynyl]benzene and 15 mL of tetrahydrofuran solution containing 0.4 mmol of tetrabutylammonium fluoride were stirred and reacted at 8°C for 10 min, then 10 mL of ethyl acetate was added, and the reaction product was washed with brine, And add 20g of anhydrous sodium sulfate to dry. Subsequently, the resulting product was dried at room temperature under vacuum until the solvent was completely evaporated, and dispersed in pyridine at a ratio of 25 mL of pyridine per 10 mg of the product, and the resulting solution was slowly added to a copper foil filled with nitrogen, and the reaction was stirred for 2 days. At this time, A graphdiyne film will appear on the surface of the copper foil. Subsequently, the copper foil was washed with 10 mL of acetone and 10 mL of di...
Embodiment 2
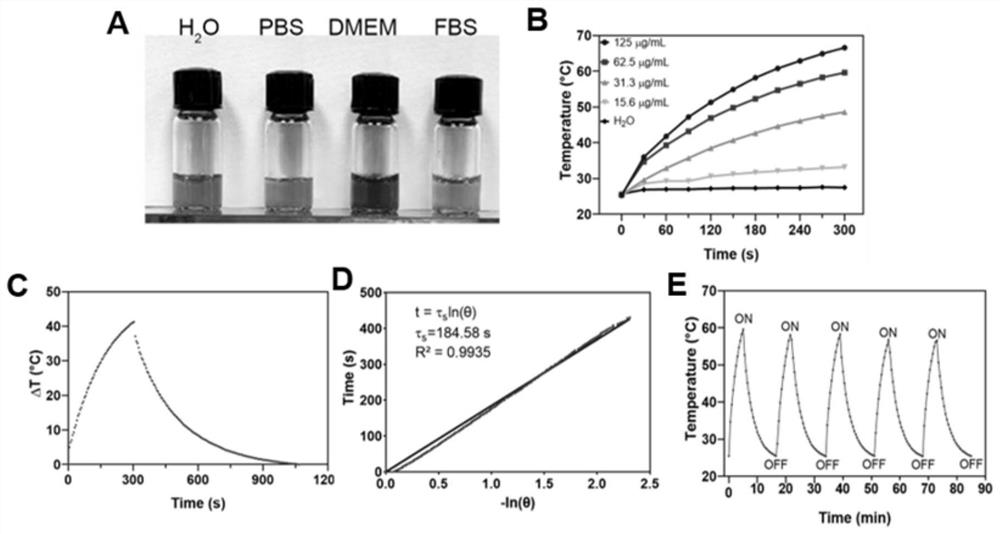
[0068] An embodiment of the present invention, this embodiment provides a photothermal behavior of PEG-GDY. Prepare PEG-GDY solutions with gradient concentrations (15.6 μg / mL, 31.3 μg / mL, 62.5 μg / mL, 125 μg / mL). Place the PEG-GDY solutions with gradient concentrations in transparent quartz dishes respectively, at 1W / cm 2 Intensity 808nm near-infrared light was irradiated for 5 minutes, and the temperature changes were recorded respectively. In addition, the light-to-heat conversion coefficient was about 32%. The calculation formula is as follows:
[0069] η=(hA△T max –Q) / I(1-10 -Aλ )
[0070] In the formula, η is the light-to-heat conversion efficiency, h is the heat transfer coefficient of dispersed light and heat, A is the surface area of the container, and ΔT max is the maximum temperature rise under near-infrared light, I is light intensity, -A 808 is the absorbance of PEG-GDY under near-infrared light irradiation, and Q is the heat absorbed by the pure dispersion l...
Embodiment 3
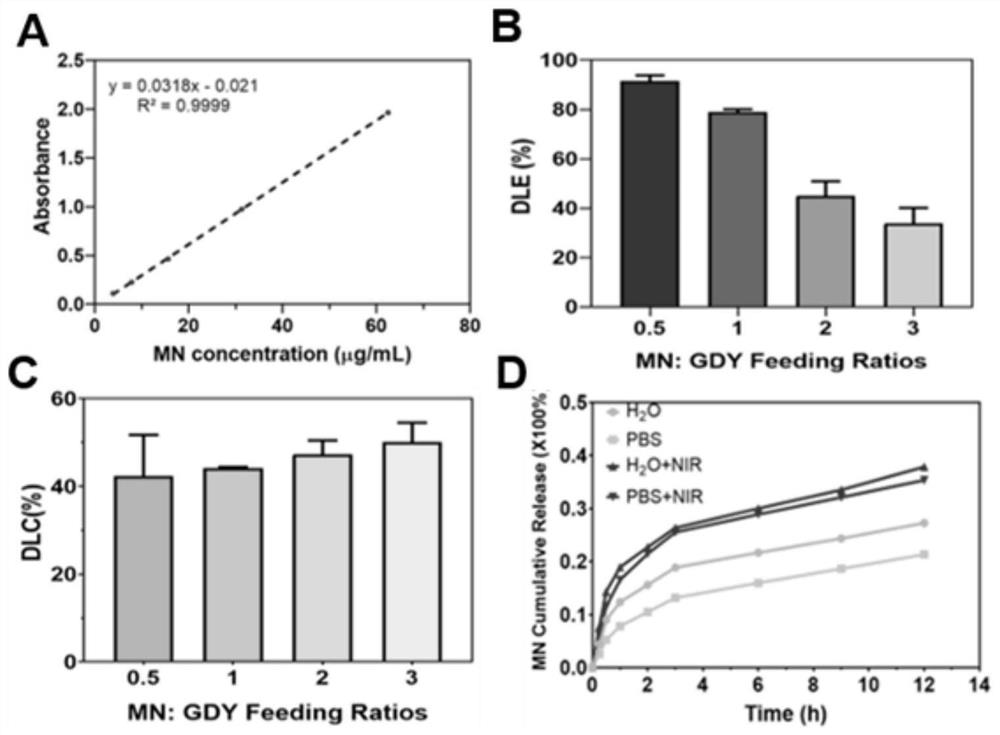
[0073] An embodiment of the present invention, this embodiment provides a method for loading MN with PEG-GDY. First prepare different proportions of MN aqueous solutions (3.91 μg / mL, 7.81 μg / mL, 15.6 μg / mL, 31.3 μg / mL, 62.5 μg / mL), measure the absorbance at 365 nm with a UV-visible spectrophotometer, and draw the concentration-absorbance standard curve line. Take 1 mg of PEG-GDY aqueous solution with a concentration of 1 mg / mL at a concentration of 1 mg / mL, and add a concentration of 1 mg / mL according to the volume ratio of PEG-GDY aqueous solution and MN aqueous solution at 1:0.5, 1:1, 1:2, and 1:3, respectively. MN aqueous solution was stirred overnight, and centrifuged at 12000rpm for 5min to obtain MN-PEG-GDY, and the MN content in the supernatant was determined to calculate the drug loading rate and drug loading amount. Depend on image 3 As shown in B and 3C, the drug loading rate of MN decreases with the increase of the feed ratio, while the drug load increases with t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com