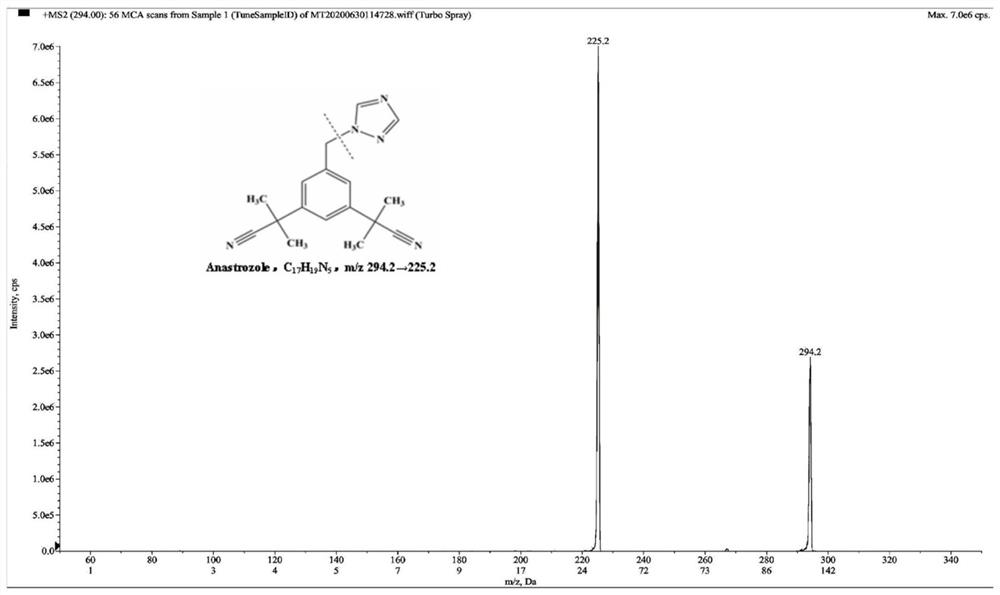
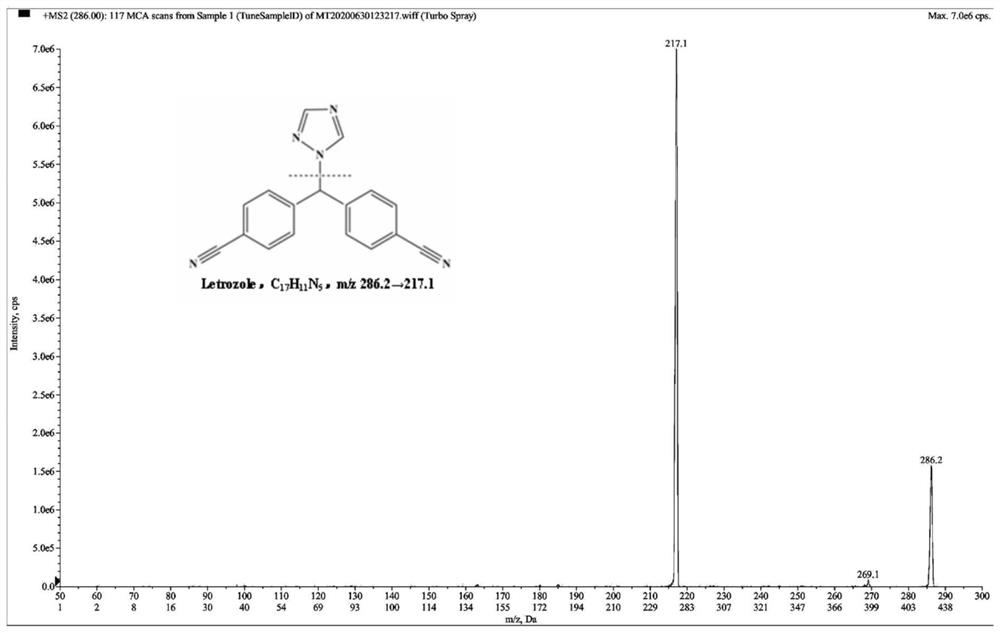
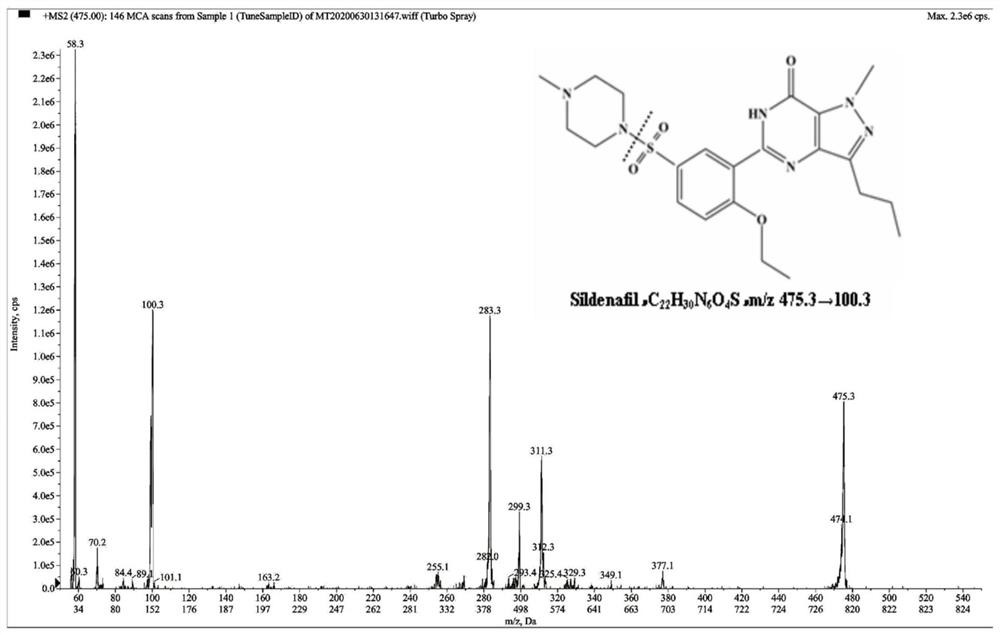
Method for simultaneously detecting concentration of aromatase inhibitor, type 5 phosphodiesterase inhibitor and metabolites of aromatase inhibitor and type 5 phosphodiesterase inhibitor in human plasma
A type of phosphodiesterase and phosphodiesterase technology, applied in the field of clinical blood drug concentration monitoring, can solve the problems of narrow linear range, high detection cost, low sensitivity, etc., achieve good precision and accuracy, and extraction recovery rate High, high-sensitivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Detection of Aromatase Inhibitors and Type 5 Phosphodiesterase Inhibitors and Their Metabolite Concentrations in Plasma
[0077] 1. Sample pretreatment
[0078] A stock solution of bifonazole was prepared with methanol as a solvent. In the stock solution of bifonazole, the concentration of bifonazole was 1 mg / mL, and it was sealed in a 4° C. refrigerator for future use.
[0079] The stock solution of bifonazole was diluted with methanol aqueous solution with a volume ratio of 1:1 to prepare an aqueous methanol solution of bifonazole, wherein the concentration of bifonazole was 0.25 μg / mL. The methanol aqueous solution of bifonazole was mixed with acetonitrile in a volume ratio of 1:20 to obtain a protein precipitant.
[0080] Take a human plasma sample, add 2 times the volume of protein precipitant, vortex for 3 min to precipitate protein, centrifuge at 12000g / min for 10 min at 4°C, take 100 μL of supernatant and put it in a sample injection tube to obtain a ...
Embodiment 2
[0100] Example 2 Quality control solution
[0101] The stock solutions of aromatase inhibitor, phosphodiesterase inhibitor type 5 and their metabolite standard products in steps 1 and 2 of Example 1 above and the stock solutions of bifonazole were diluted with an aqueous methanol solution with a volume ratio of 1:1. Stock solutions, respectively obtain quality control solutions of different concentrations of each component and internal standard, and mix the quality control solutions of different concentrations according to each concentration gradient to obtain a mixed quality control solution.
[0102] Take a series of 90 μL blank plasma samples, add 10 μL mixed quality control solution of a certain concentration gradient respectively, and use step 2 of the above Example 1 to process, and sequentially prepare quality control plasma solutions of different concentrations. The quality control plasma solutions are quantitative The lower limit LLOQ, the low quality control LQC, the...
Embodiment 3
[0105] Example 3 Methodological verification
[0106] Methodological validation of the method in the present invention mainly includes selectivity, linearity, precision and accuracy, matrix effect and recovery rate, and stability.
[0107] 1. Selective
[0108] 100 μL of human blank plasma sample was taken and processed according to step 1 of Example 1, wherein the methanol aqueous solution of bifonazole was not added to the protein precipitation agent. Take 100 μL of LLOQ plasma sample and process according to step 1 of Example 1. Then, the human blank plasma sample and the LLOQ plasma sample were tested according to step 3 of Example 1. It can be found that anastrozole, letrozole, sildenafil, N-desmethylsildenafil, tadalafil and bifonazole have good peak shapes under the above chromatographic conditions. For blank plasma, the peak area of the interference signal in blank plasma was compared with the peak area of analyte and internal standard in LLOQ.
[0109] 2. Line...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com