Stable compound famotidine chewable tablet and preparation method thereof
A technique for famotidine and chewable tablets, which is applied in the field of preparation of compound famotidine chewable tablets and compound famotidine chewable tablets, which can solve the problems of sustained release of famotidine and ease of chewing and taking. , to achieve good chewability, easy industrial production, and low friability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
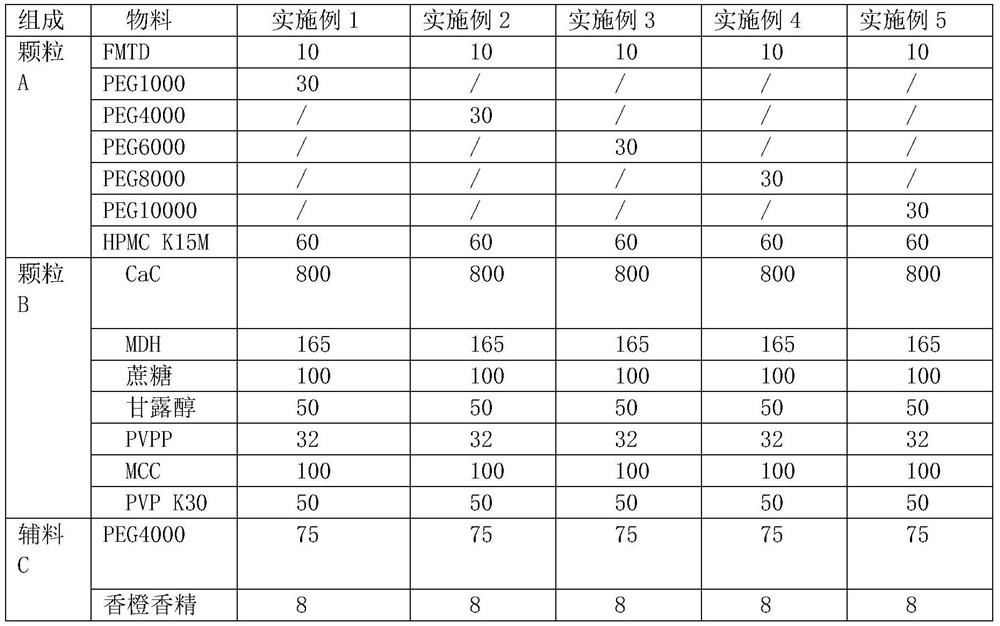
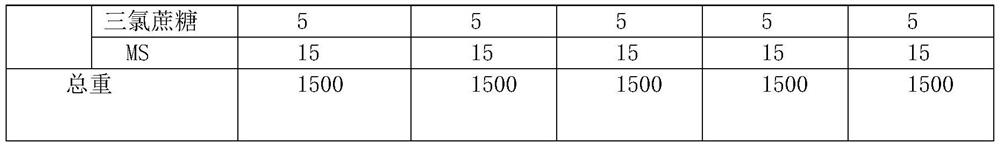
[0038] Table 1 embodiment 1-5 prescription composition (mg / tablet, 500 tablets / batch)
[0039]
[0040]
[0041] Example 1-5 Preparation process: Weigh the material of granule A according to the prescription, mix, heat to 70°C, stir evenly, let cool to room temperature, crush and pass through a 20-mesh sieve to obtain granule A. Weigh PVP K30 according to the prescription, add water to dissolve and make 10% PVPK30 solution as the binder solution, weigh other materials of granule B according to the prescription, mix evenly, add the binder solution to make soft material, pass through a 20-mesh sieve to granulate, 60 ℃ drying, passing through 18-mesh sieve and sizing to obtain granule B. Mix granules A, granules B and excipients C evenly, control the tablet hardness to 60N, and press into tablets to obtain chewable tablets of this product.
Embodiment 6-11
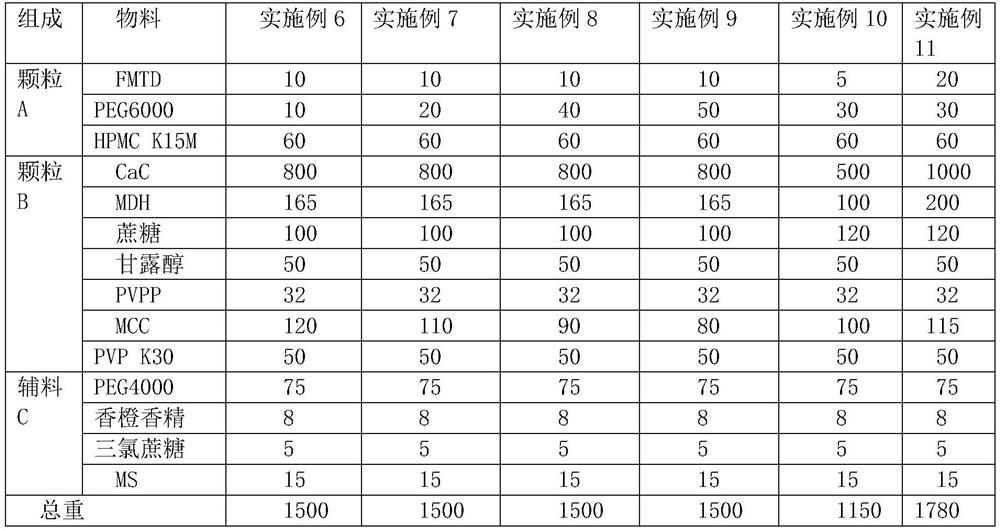
[0043] Table 2 embodiment 6-11 prescription composition (mg / tablet, 500 tablets / batch)
[0044]
[0045]Example 6-11 Preparation process: Weigh the material of granule A according to the prescription, mix, heat to 70°C, stir evenly, let cool to room temperature, crush and pass through a 20-mesh sieve to obtain granule A. Weigh PVP K30 according to the prescription, add water to dissolve and make 10% PVPK30 solution as the binder solution, weigh other materials of granule B according to the prescription, mix evenly, add binder solution to make soft material, pass through a 20-mesh sieve to granulate, 60 ℃ drying, passing through 18-mesh sieve and sizing to obtain granule B. Mix granules A, granules B and excipients C evenly, control the tablet hardness to 60N, and press into tablets to obtain chewable tablets of this product.
Embodiment 12-15
[0047] Table 3 embodiment 12-15 prescription composition (mg / tablet, 500 tablets / batch)
[0048]
[0049] Examples 12-15 Preparation process: Weigh the material of granule A according to the prescription, mix, heat to 70°C, stir evenly, let cool to room temperature, crush and pass through a 20-mesh sieve to obtain granule A. Weigh PVP K30 according to the prescription, add water to dissolve and make 10% PVPK30 solution as the binder solution, weigh other materials of granule B according to the prescription, mix evenly, add binder solution to make soft material, pass through a 20-mesh sieve to granulate, 60 ℃ drying, passing through 18-mesh sieve and sizing to obtain granule B. Mix granules A, granules B and excipients C evenly, control the tablet hardness to 60N, and press into tablets to obtain chewable tablets of this product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com