Novel asymmetric catalytic nucleophilic fluorine heterocyclization reaction system and application thereof in chiral non-natural amino acid module synthesis
An unnatural amino acid, asymmetric technology, applied in the field of new asymmetric catalyzed nucleophilic fluorine heterocyclization reaction system, to achieve the effect of environmental friendliness, mild reaction conditions and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 Establishment of a novel asymmetric catalytic nucleophilic fluorine heterocyclization reaction system
[0094] The novel asymmetric catalytic nucleophilic fluorine heterocyclization reaction system includes chiral iodine catalyst CIC1 and boron trifluoride diethyl ether (BF 3 ·Et 2 O).
[0095] Wherein, the preparation method of the centrosymmetric chiral iodine catalyst CIC1 is as follows:
[0096] Synthesized according to the method in the reference (Banik, S.M.; Medley, J.W.; Jacobsen, E.N. Catalytic, Asymmetric Difluorination of Alkenes to Generate Difluoromethylated Stereocenters. Science 2016, 353, 51-54).
[0097] NMR characterization: 1 H NMR (500MHz, CDCl 3 )δNMR (500MHz, CDCl, J.W.; Jacobsen, E.N.Catalytic, Asymmetric Difluorination of Alkenes to Generate Difluoromethylated Stereocenters. Science 2016, 353, 51-54 Fluorine reagent, cheap and easy to get, good stability, low toxicity, is currently the cheapest fluorine in the market Reagent; mild ...
Embodiment 2
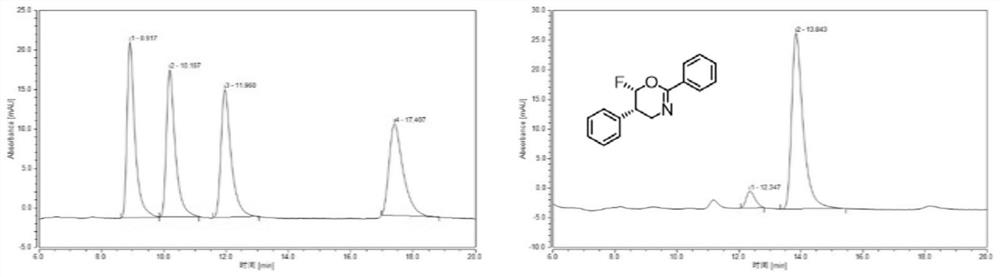
[0098] Example 2 Catalytic asymmetric nucleophilic fluoroheterocyclization of N-cinnamylbenzamide (1a) to generate chiral fluorooxazine derivatives
[0099] The catalytic asymmetric nucleophilic fluorine heterocyclization reaction in this embodiment is carried out according to the following reaction formula:
[0100]
[0101] The specific operation method is:
[0102] (1) In a 15mL reaction tube, add 1a (47.4mg, 0.2mmol, 1.0equiv.), catalyst CIC1 (20mol%) and 8mL DCE, cool down to -25°C and keep it at this temperature for 10 minutes, then add m-CPBA (0.24mmol, 1.2equiv.) and slowly drop into BF 3 ·Et 2 O (2 mmol, 10.0 equiv.). After the dropwise addition, the stopper of the reaction tube was tightened, and the stirring was continued at -25°C for 48 hours. After the reaction was completed, the reaction liquid was poured into 10 mL of saturated sodium bicarbonate solution, shaken in a separatory funnel and left to stand for layering, and the organic phase was continued to...
Embodiment 3
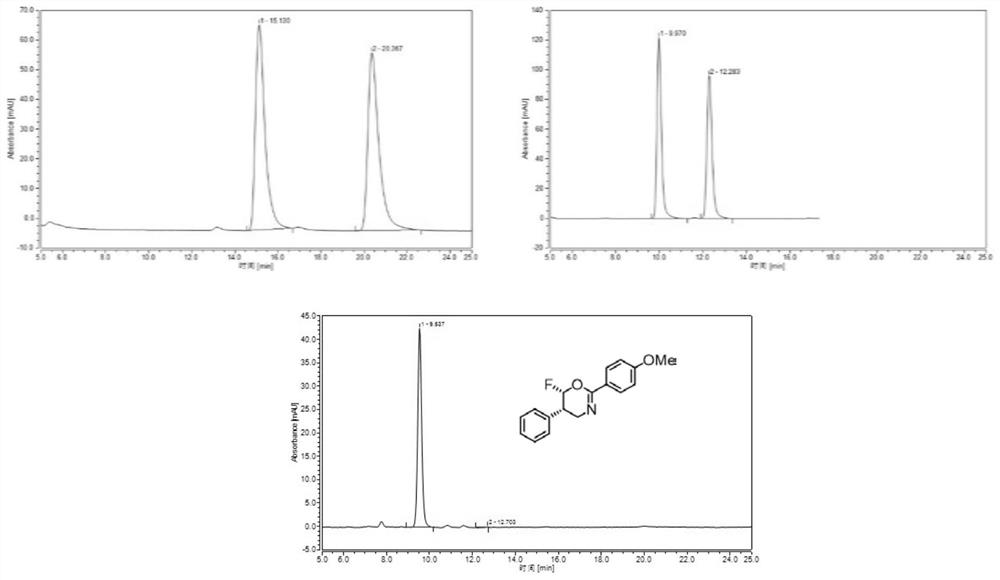
[0110] Example 3 Catalytic asymmetric nucleophilic fluoroheterocyclic reaction of N-cinnamyl-4-methoxybenzamide (2a) to generate chiral fluorooxazine derivatives
[0111] The catalytic asymmetric nucleophilic fluorine heterocyclization reaction in this embodiment is carried out according to the following general reaction formula:
[0112]
[0113] The specific operation method is:
[0114] (1) Add 2a (53.5 mg, 0.2 mmol, 1.0 equiv.), catalyst CIC1 (20 mol%) and 8 mL of DCE into a 15 mL reaction tube, cool down to -25 ° C and keep at this temperature for 10 minutes, and then add m-chlorine Peroxybenzoic acid (m-CPBA, 0.24mmol, 1.2equiv.), and slowly drop into BF 3 ·Et 2 O (2 mmol, 10.0 equiv.). After the dropwise addition, the stopper of the reaction tube was tightened, and the stirring was continued at -25°C for 24 hours. After the reaction was completed, the reaction solution was poured into 10 mL of saturated sodium bicarbonate solution, shaken in a separatory funnel a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com