Preparation method of nanometer flaky manganese oxide material and application of nanosheet-shaped manganese oxide material in aqueous zinc ion battery
A nano-flaky, manganese oxide technology, applied in manganese oxide/manganese hydroxide, battery electrodes, nanotechnology, etc., can solve the problems of high cost and unsuitable for large-scale mass production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Preparation of solution: Dissolve 0.02mol of manganese chloride tetrahydrate in 96mL of absolute ethanol, then add 4mL of 30wt% aqueous hydrogen peroxide and mix evenly to obtain a mixed ethanol solution of manganese chloride and hydrogen peroxide. Wherein, the ethanol concentration of manganese chloride is 0.2M, and the ethanol concentration of hydrogen peroxide is 0.4M. Subsequently, 0.04 mol of sodium hydroxide was dissolved in 40 mL of absolute ethanol to obtain a 1M ethanol solution of sodium hydroxide.
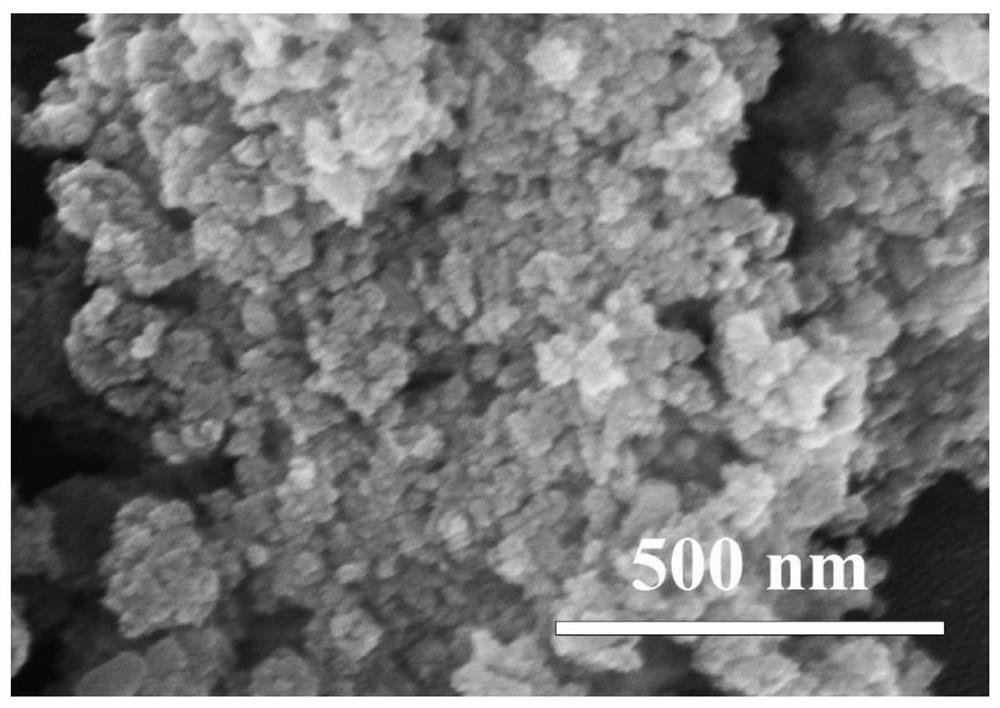
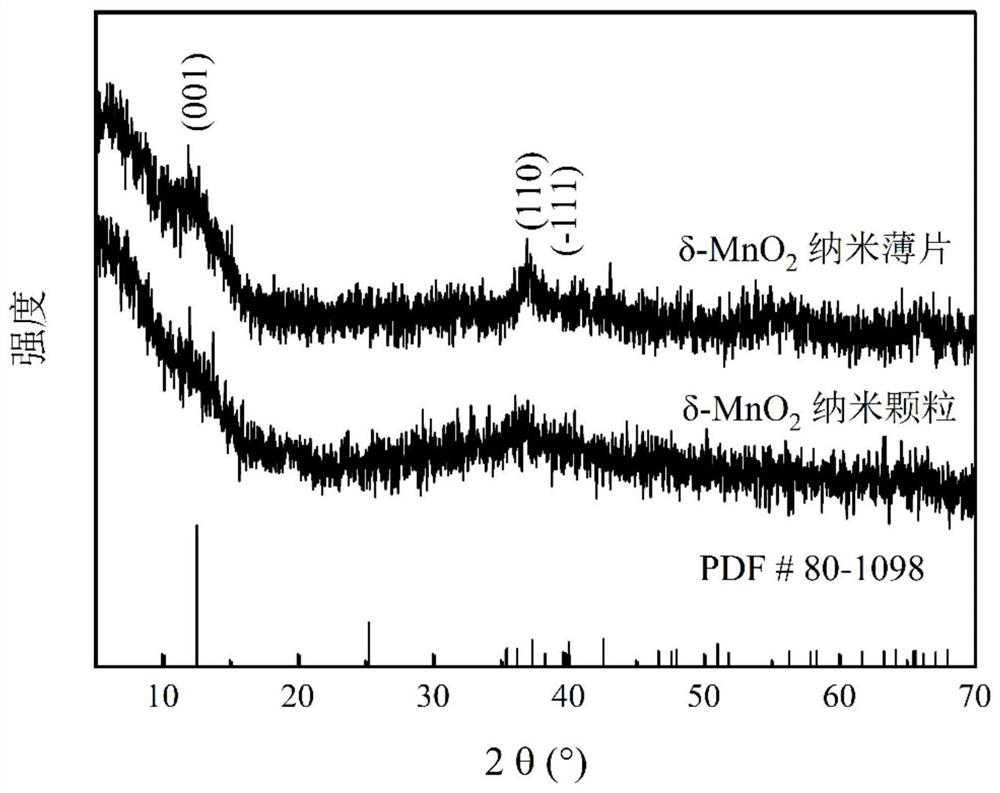
[0033] 2) Preparation of nanoparticle-like δ-MnO 2 : At room temperature and normal atmospheric pressure, the sodium hydroxide ethanol solution is mixed with the ethanol solution mixed with manganese chloride and hydrogen peroxide in stirring to carry out coprecipitation reaction, wherein the divalent manganese ion, sodium hydroxide and hydrogen peroxide The amount ratio of substances is 1:2:2. After the reaction was over, the stirring was continued for 6h. ...
Embodiment 2
[0037] 1) Preparation of solution: Dissolve 0.04mol of manganese nitrate tetrahydrate in 88mL of absolute ethanol, then add 12mL of 30wt% hydrogen peroxide aqueous solution and mix evenly to obtain a mixed ethanol solution of manganese nitrate and hydrogen peroxide. Wherein, the ethanol concentration of manganese nitrate is 0.4M, and the ethanol concentration of hydrogen peroxide is 1.2M. Subsequently, 0.08 mol of sodium hydroxide was dissolved in 40 mL of absolute ethanol to obtain a 2M ethanol solution of sodium hydroxide.
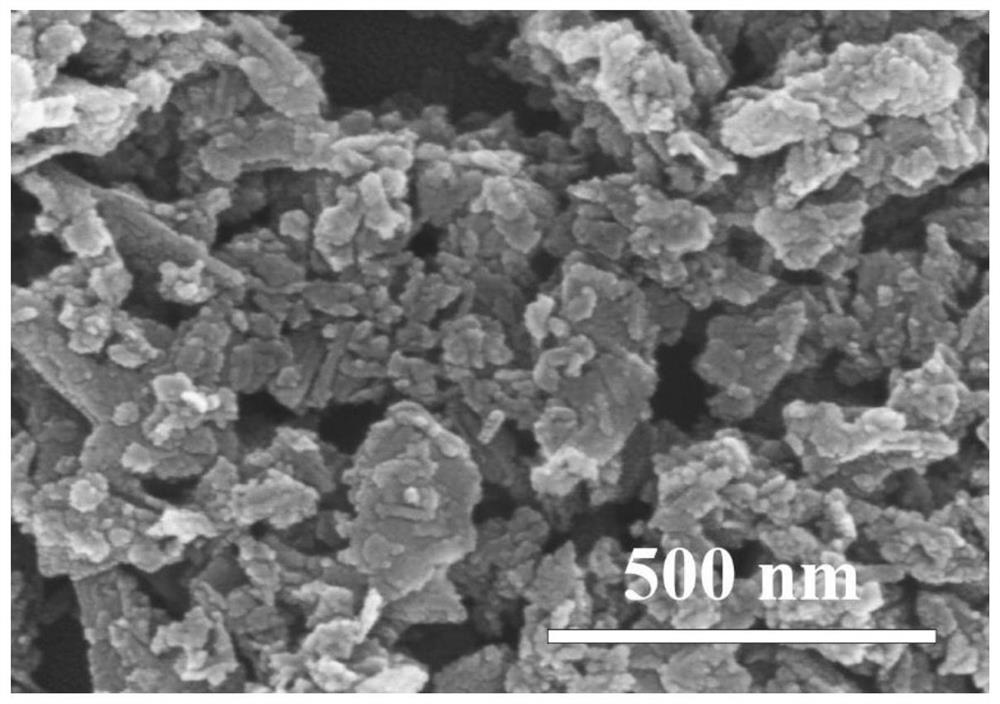
[0038] 2) Preparation of nanoparticle-like δ-MnO 2 : At room temperature and normal atmospheric pressure, mix the ethanol solution of sodium hydroxide with the ethanol solution mixed with manganese nitrate and hydrogen peroxide in stirring, and carry out coprecipitation reaction, wherein the substances of divalent manganese ions, sodium hydroxide and hydrogen peroxide The amount ratio is 1:2:3. After the reaction was completed, stirring was continued f...
Embodiment 3
[0042] 1) Preparation of solution: Dissolve 0.03 mol of manganese sulfate tetrahydrate in 94 mL of absolute ethanol, then add 6 mL of 30 wt % aqueous hydrogen peroxide and mix well to obtain a mixed ethanol solution of manganese sulfate and hydrogen peroxide. Wherein, the ethanol concentration of manganese sulfate is 0.3M, and the ethanol concentration of hydrogen peroxide is 0.6M. Subsequently, 0.06 mol of sodium hydroxide was dissolved in 40 mL of absolute ethanol to obtain a 1.5 M sodium hydroxide ethanol solution.
[0043] 2) Preparation of nanoparticle-like δ-MnO 2 : At room temperature and normal atmospheric pressure, the ethanol solution of sodium hydroxide and the ethanol solution mixed with manganese sulfate and hydrogen peroxide are mixed for coprecipitation reaction, wherein the substances of divalent manganese ions, sodium hydroxide and hydrogen peroxide The amount ratio is 1:2:2. After the reaction was over, the stirring was continued for 10 h. The precipitate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com