Fluorescent probe of chalcone structure and its preparation method and application of detecting hydrazine
A fluorescent probe, chalcone technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of long synthesis route, low yield, unsatisfactory detection, etc. Ease of handling and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
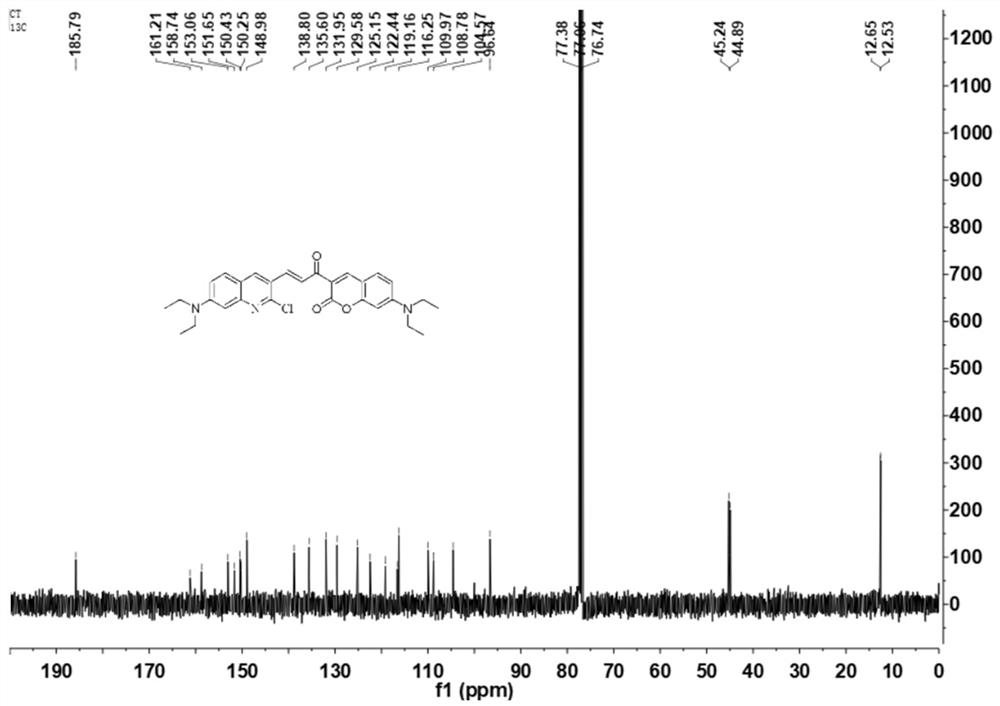
[0028] Preparation of 3-(3'-(2'-chloro-7'-diethylaminoquinoline propenone-7-diethylaminocoumarin (QCT-Cl) Weigh 2-chloro-7-diethylaminoquinone Add 7-diethylaminocoumarin-3-ethanone to a 100mL two-neck bottle, add solvent, stir to dissolve, then add catalyst, the solution is reddish brown, heat up to 75°C, and detect the reaction by TLC Process. After the completion of the reaction, cool to room temperature to precipitate an orange solid, pressurize suction filtration, and wash with ethanol to obtain an orange solid, which is 3-(3'-(2'-chloro-7'-diethylaminoquinoline propene Keto-7-diethylaminocoumarin.
[0029] In the above-mentioned preparation method, some conditions are changed, and the yield effect is as follows:
[0030]
[0031]
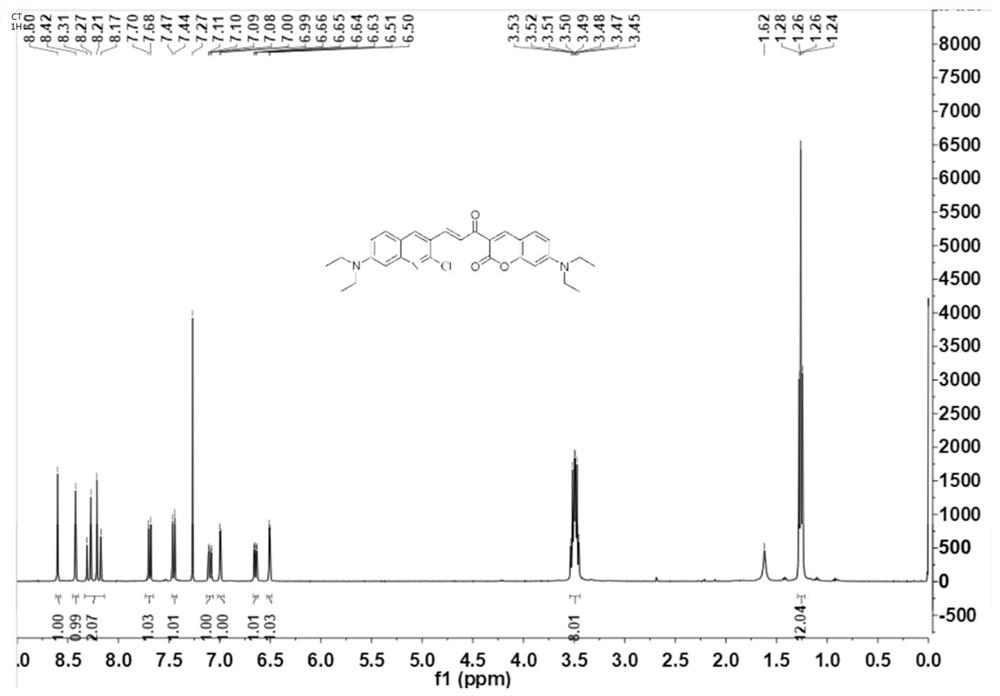
[0032] Example 1-1 obtained 4.12 g of orange solid with a yield of 82%. m.p.:212-213℃. 1 H NMR (400MHz, CDCl 3 )δ8.60(s,1H),8.42(s,1H),8.24(dd,J=39.2,15.6Hz,2H),7.69(d,J=9.2Hz,1H),7.45(d,J=4.4 Hz,1H),7.10(dd,J=9.2,2.8Hz,1H),6.99(d,J=...
Embodiment 2
[0033] The preparation of embodiment 2 test solutions
[0034] (1) Preparation procedure of stock solution:
[0035] In a 10 mL vial, make up 10 in DMSO -3 The mol / L QCT-Cl stock solution is ready for use. In a 2mL sample bottle, add 0.2mL double-distilled water, then add 1.8mL DMSO, and then add 0.01mol / L of the hydrazine standard solution (20μL, 10 -2 mol / L), mix well; finally add 20 μL of QCT-Cl in DMSO solution (10 -3 mol / L), and mix again. After standing for 0.5 hours, measure its fluorescence emission spectrum (380nm is the excitation wavelength). The above operation, without adding the sample solution to be tested, is the preparation of the blank test solution.
[0036] (2) Fluorescence spectrum test:
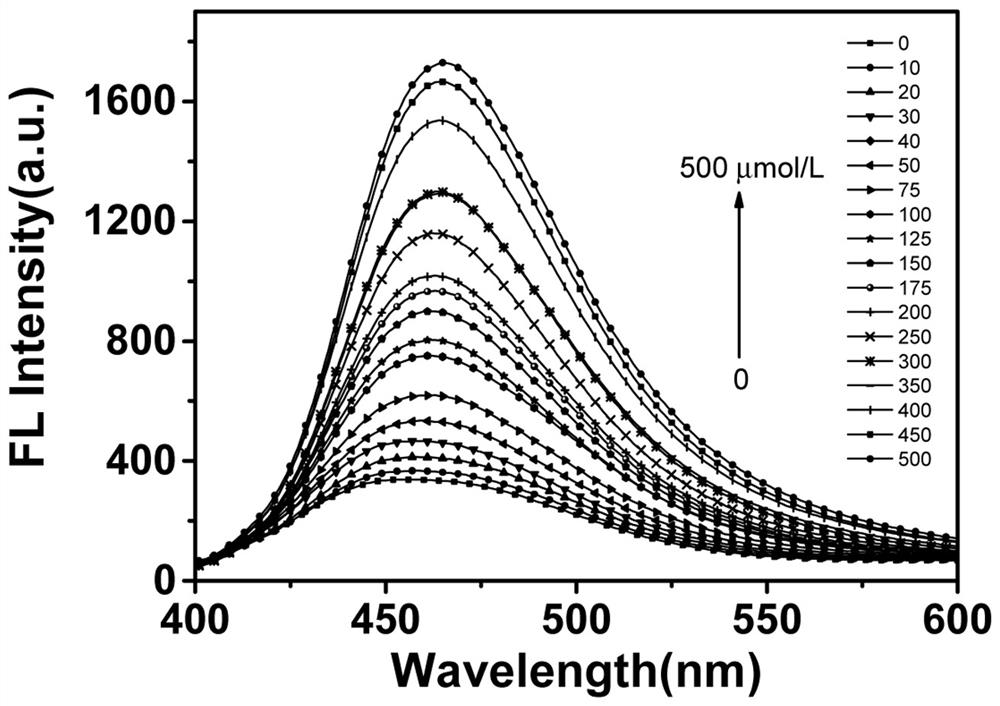
[0037] The blank test solution of QCT-Cl has a weak fluorescence emission peak at 460nm; when hydrazine exists, the fluorescence at 460nm increases; and with the increase of hydrazine concentration, the intensity increases significantly, see image 3 .
[0038] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com