Application of Akkermansia muciniphila in the aspect of treating chronic pancreatitis
A technology for chronic pancreatitis and pancreatic atrophy, applied in the direction of application, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve unclear problems, reduce release, broaden application fields, and relieve inflammation The effect of infiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Akkermansia muciniphila bacterial agent preparation
[0028] Preparation of active bacterial agents: Akkermansia muciniphila BAA-835 was cultured strictly anaerobically in brain heart infusion medium supplemented with 2% (m / v) type III mucin. Centrifuge the bacterial solution in the logarithmic growth phase at 4°C and 8000g for 10 minutes, remove the supernatant, wash the bacterial sludge twice with sterile physiological saline, and resuspend the bacterial sludge in glycerol with a volume fraction of 30% (v / v) , optionally, it can be stored in a -80°C refrigerator.
[0029] Preparation of inactivated bacteria: Akkermansia muciniphila BAA-835 was cultured strictly anaerobically in brain heart infusion medium supplemented with 2% (m / v) type III mucin. Centrifuge the bacterial solution in the logarithmic growth phase at 4°C and 8000g for 10 minutes, remove the supernatant, wash the bacterial sludge twice with sterile physiological saline, and resuspend the ba...
Embodiment 2
[0031] Embodiment 2 The preparation of the medicine containing Akkermansia muciniphila
[0032] The bacterial suspension containing Akkermansia muciniphila BAA-835 living cells or inactivated cells prepared in Example 1 is mixed with the auxiliary materials to obtain a liquid preparation; optionally, compression molding is made into tablets; alternatively, the liquid The formulation is dried to obtain a powder; alternatively, the powder is used as a filling to prepare capsules.
[0033] Wherein, the pharmaceutical excipients include anti-adhesives, penetration enhancers, buffers, plasticizers, surfactants, defoamers, thickeners, clathrates, absorbents, humectants, solvents, propellants, Solvents, solubilizers, emulsifiers, colorants, pH regulators, binders, disintegrants, fillers, lubricants, wetting agents, integrating agents, osmotic pressure regulators, stabilizers, glidants, flavorings additives, preservatives, foaming agents, suspending agents, coating materials, fragran...
Embodiment 3
[0034] Example 3 Akkermansia muciniphila BAA-835 for the treatment of chronic pancreatitis
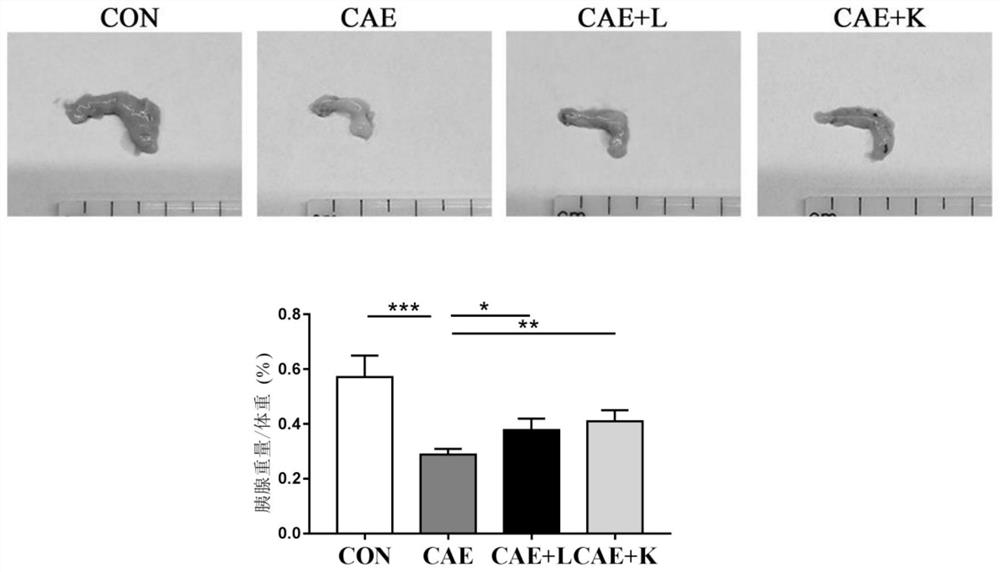
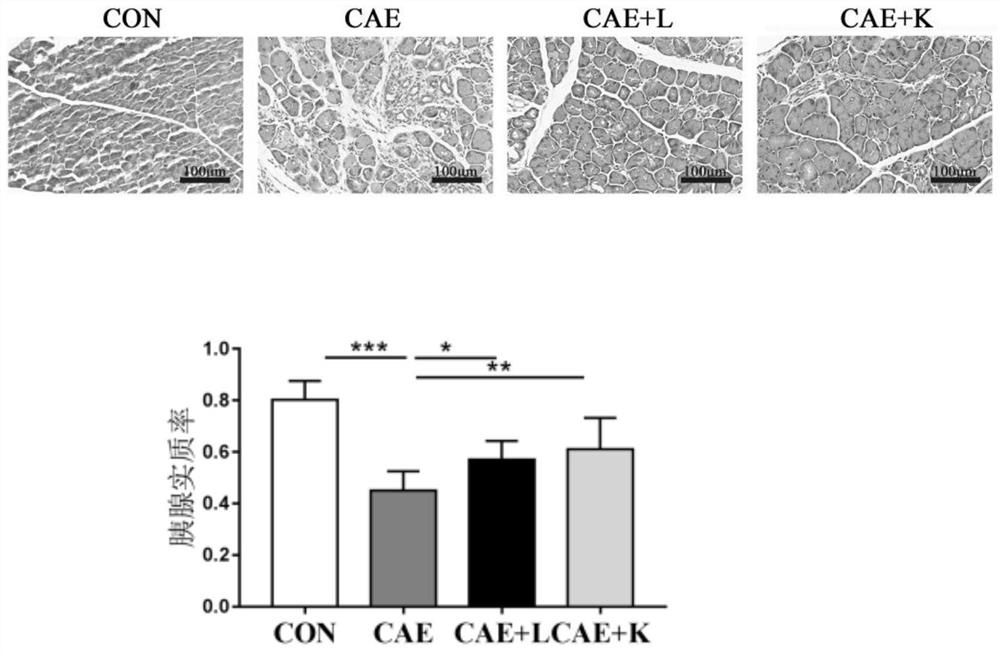
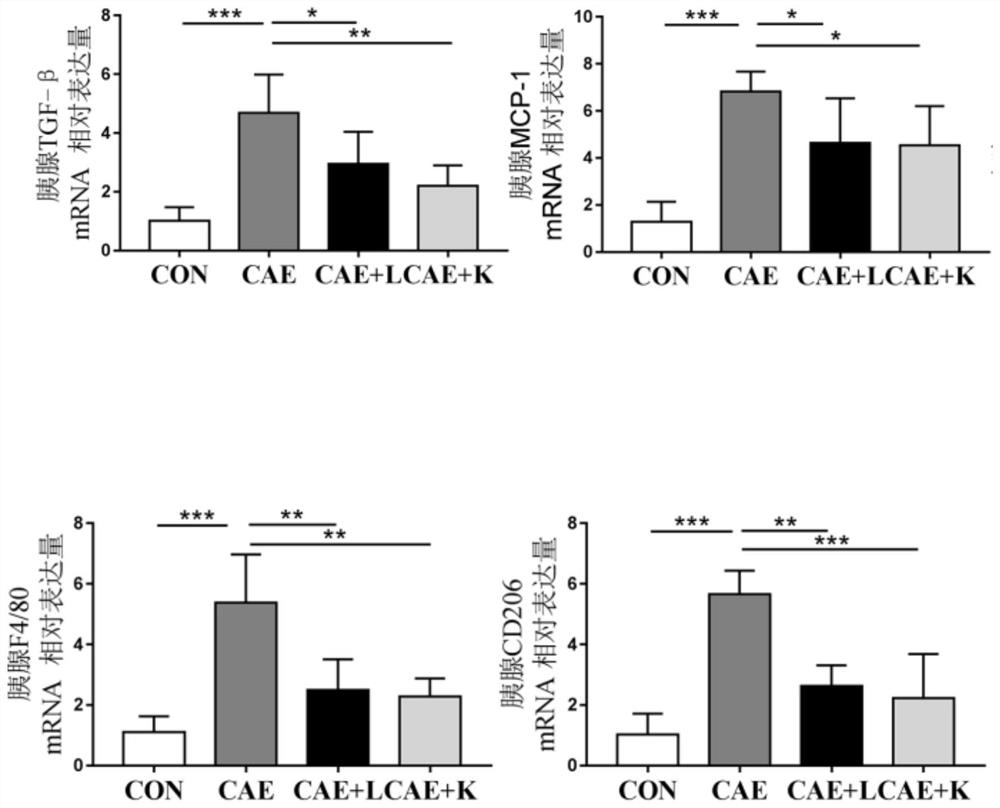
[0035] (1) Establishment of chronic pancreatitis model:
[0036] 8-week-old 20-23g female C57BL / 6J mice were randomly divided into control group (CON), inflammation model group (CAE), Akkermansia muciniphila BAA-835 live bacteria treatment group (CAE+L), Akkermansiamuciniphila BAA-835 Pasteurized inactivated bacteria treatment group (CAE+K), 8 animals in each group, provided controlled feeding conditions. The chronic pancreatitis model was induced by intraperitoneal injection of cerulein. Dissolve cerulein in normal saline, inject cerulein (50 μg / kg body weight) every hour for 6 consecutive injections, three days a week for four weeks. Mice in the CON group were given an equal volume of saline. During the modeling period, each mouse in the CAE+L group was given 5×10 3% glycerol by intragastric administration every day. 9CFU / mL of Akkermansia muciniphila live bacteria solution 200 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com