Glycoamine Amadori derivative as well as preparation method and application thereof
A technology of derivatives and sugar amines, applied in the field of sugar amine Amadori derivatives and their preparation, can solve the problems of high price of Amadori compounds, lack of means for regulating their degradation products, complex types of thermal degradation products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The present invention provides a method for preparing the sugar amine Amadori derivative described in the above technical scheme, comprising the following steps:
[0027] (1) fructose, acetone and vitriol oil are mixed, carry out condensation reaction, obtain protected fructose;
[0028] (2) Mix the protected fructose obtained in the step (1) with trifluoromethanesulfonic anhydride, 2,6-di-tert-butyl-4-picoline and anhydrous dichloromethane, and carry out a substitution reaction to obtain intermediate body;
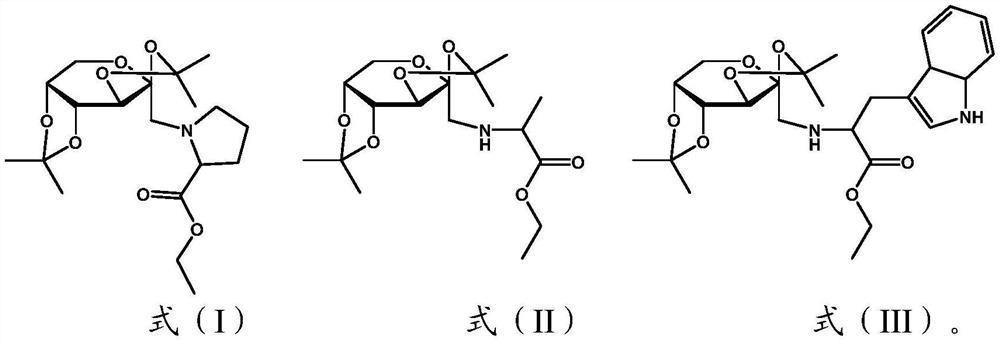
[0029] (3) mixing the intermediate obtained in the step (2) with amino acid ester, N,N-diisopropylethylamine and anhydrous dimethylformamide, and performing a substitution reaction to obtain sugar amine Amadori derivatives; The amino acid esters include proline esters, alanine esters or tryptophan esters.
[0030] The invention mixes fructose, acetone and concentrated sulfuric acid for condensation reaction to obtain protected fructose. In the present invention,...
Embodiment 1
[0084] (1) Mix 11.1mmol fructose, 39mL acetone and 36.4mmol concentrated sulfuric acid (the ratio of the amount of fructose, acetone and concentrated sulfuric acid is 1:47.7:3.28), stir in an ice-water bath for 4h to carry out condensation reaction, to the condensation reaction Add 27.8 mL of ice NaOH solution with a concentration of 5.5 mol / L to the final system, extract 8 times with 30 mL of anhydrous dichloromethane, dry the extract over anhydrous magnesium sulfate, filter and concentrate under reduced pressure to obtain protected fructose;
[0085] (2) Under nitrogen protection, 2.3mmol protected fructose, 2.3mmol trifluoromethanesulfonic anhydride and 1.5mmol 2,6-di-tert-butyl-4-picoline were added to 10mL of anhydrous dichloromethane (protected The ratio of the amount of fructose to trifluoromethanesulfonic anhydride, 2,6-di-tert-butyl-4-picoline and anhydrous dichloromethane is 1:1:0.65:67.8), reacted in ice-water bath for 1h , carry out the substitution reaction, after...
Embodiment 2
[0090] The difference from Example 1 is that the proline ester is replaced by alanine ester, and the remaining steps are the same as in Example 1, and the yield is 80%.
[0091] The sugar amine Amadori derivatives prepared in this example have the structure shown in formula (II).
[0092] The H NMR spectrum of the sugar amine Amadori derivatives prepared in this embodiment is: H NMR spectrum: 1 H NMR (400MHz, CDCl 3 , containing 0.03% TMS, 25°C): δ: 4.58 (dd, J = 7.9Hz, J = 2.6Hz, 1H, CH), 4.34 (d, J = 2.5Hz, 1H, CH), 4.22 (dd, J =7.9Hz,J=1.0Hz,1H,CH),4.16(q,J=6.8Hz,2H,CH 2 ),3.81(dd, 2 J=60.2Hz, 3 J=13.0Hz, 2H, CH 2 ), 3.49(q, J=7.0Hz, 1H, CH), 2.85(m, 2H, CH 2 ),1.53(s,3H,CH 3 ),1.47(s,3H,CH 3 ),1.43(s,3H,CH 3 ),1.34(s,3H,CH 3 ),1.29-1.25(m,6H,2CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com