Near-infrared fluorescent probe, preparation and application of near-infrared fluorescent probe in detection of transthyroxine tetramer protein
A fluorescent probe, thyroxine technology, applied in the field of biomedicine, can solve the problems of complex inspection methods, high cost, and great physical damage, and achieve the effects of avoiding the interference of self-luminescence, long emission wavelength, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
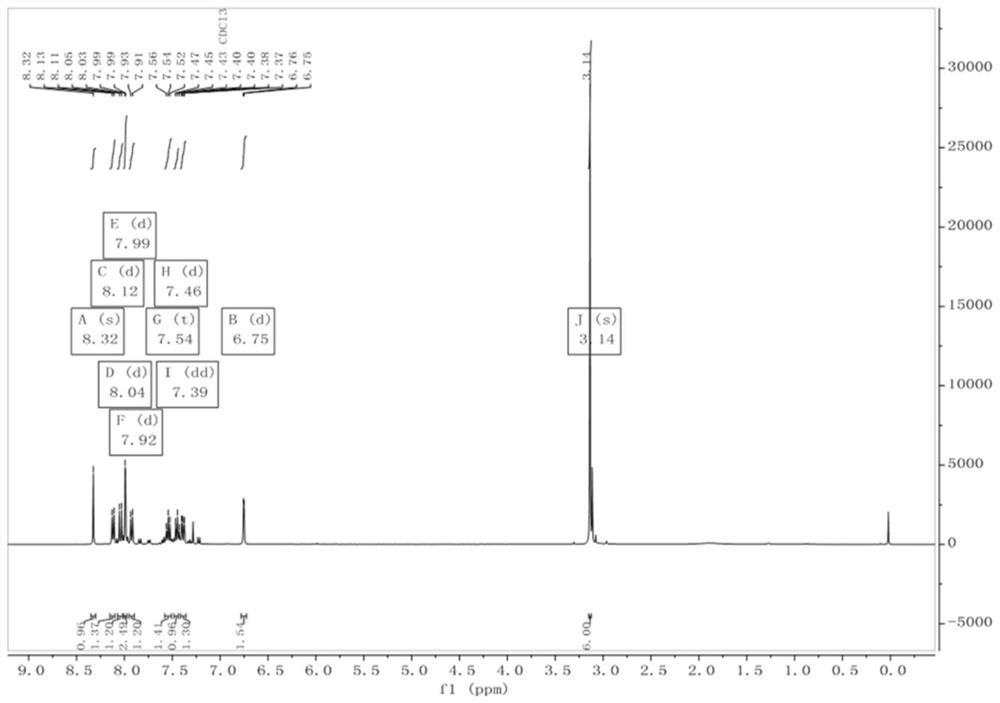
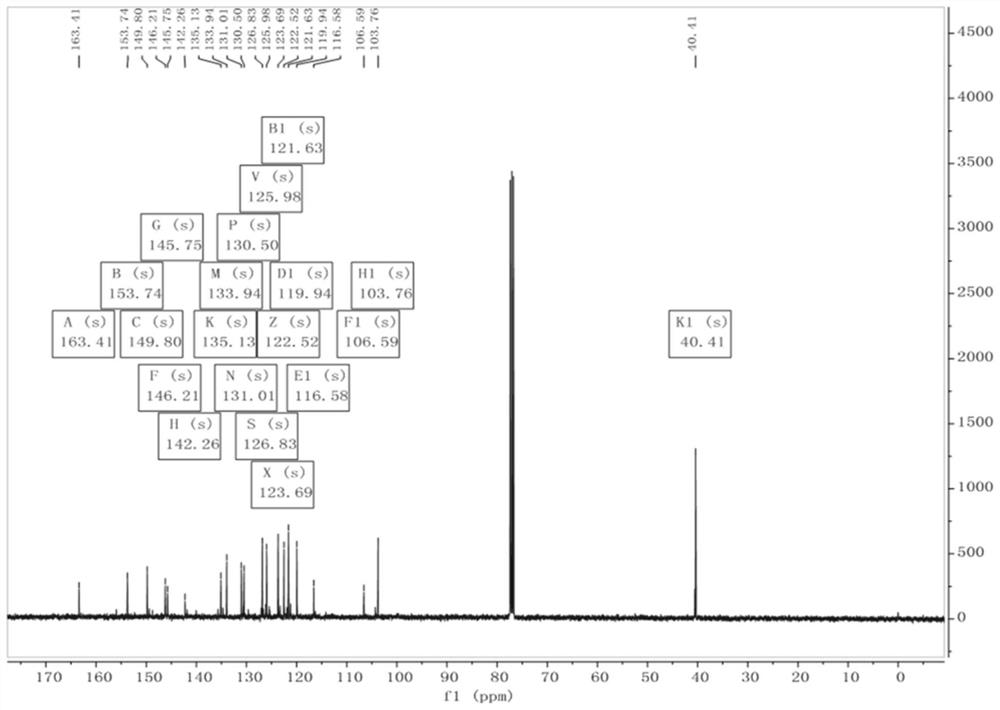
Embodiment 1
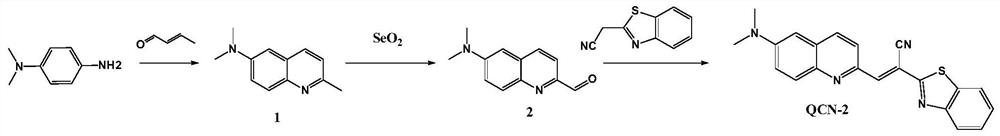
[0062] The synthesis of fluorescent probe QCN-2, its synthetic route is as follows figure 1 Shown:
[0063] (1) Synthetic steps of intermediate compound 1
[0064] First prepare the hydrochloric acid solution (HCl:H 2 O=1:1, v / v) 250ml, stir evenly, add 5.8g (42.6mmol) of N,N-dimethyl-1,4-p-phenylenediamine, after stirring evenly, add 7ml of crotonaldehyde, mix well Then, 30 ml of toluene was added to the reaction liquid, and the reaction was refluxed for 7 h under the protection of argon at 115° C. (oil bath). Thin-layer chromatography confirmed that the reaction was complete, the reaction was stopped, and the reaction was allowed to stand at room temperature. First remove the upper toluene solution, then adjust the pH to neutral with a NaOH saturated solution under ice-water bath conditions, then add an equal volume of dichloromethane (DCM) to extract at least three times, and finally separate and purify by column chromatography (silica gel 100-200 mesh; Petroleum ether:...
Embodiment 2
[0080] Fluorescent Spectrum of Fluorescent Probe QCN-2 Responding to Transthyretin Tetrameric Protein
[0081] The fluorescent probe QCN-2 (14.4 μM), the fluorescent probe QCN-2 (14.4 μM) and the wild-type transthyretin tetramer were respectively measured in 1 × PBS buffer solution (pH 7.4) by a fluorescence spectrophotometer Protein WT-TTR (14.4 μM) was co-incubated for 1 hour, and fluorescent probe QCN-2 (14.4 μM), wild-type transthyretin tetramer protein WT-TTR (14.4 μM) and guanidine hydrochloride (Guanidine Hydrochloride; 6M) Co-incubate the fluorescence emission spectrum for 1 hour, and record its fluorescence intensity value. The excitation wavelength is 484nm. Among them, the wild-type transthyretin tetramer protein WT-TTR according to the reference (Themost pathogenic transthyretin variant, L55P, forms amyloid fibrils under acidic conditions and protofilaments under physiological conditions [J].Biochemistry,1999,38(41):13560- 13573.) Purified by the method.
[0082...
Embodiment 3
[0084] Excitation and emission wavelengths of fluorescent probe QCN-2:
[0085]Compare the fluorescent probe QCN-2 prepared by the present invention with the existing fluorescent probe compound (structural formula shown in 1-7), detect the excitation and emission wavelength of the fluorescent probe; wherein, the fluorescent probe compound 1-7, As well as the acquisition of excitation and emission wavelengths, please refer to: Chem.Commun., 2019, 55, 10424—10427 (compound 1); Chem.Commun., 2013, 49, 9188—9190 (compound 2); J.Am.Chem. Soc.2015,137,7404-7414 (compound 3); J.Am.Chem.Soc.2010,132,16043–16051 (compound 4); ACS Chem.Neurosci.2016,7,924-940 (compound 5); .Am.Chem.Soc.2013, 135, 17869-17880 (Compounds 6, 7).
[0086] The results are shown in Table 1: the excitation wavelength of the fluorescent probe QCN-2 prepared in the present invention is 484nm, and the emission wavelength reaches 690nm, while the emission wavelength of the fluorescent probe compound 1-7 is shorte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com