Preparation method of artificially synthesized (R, S)-nicotine salt
A nicotine salt, artificial synthesis technology, applied in the preparation of organic compounds, carboxylates, carboxylates and other directions, can solve the problems of many steps, increased costs, expensive reagents and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
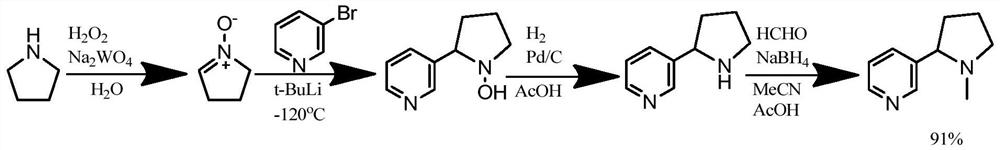
[0051]This embodiment provides an artificial synthesis (R, S)-nicotine salt, prepared according to the following steps:
[0052]S1, Take 4-methyl amino-1- (3-pyridine)-ketone hydrochloride (13.8 g, 0.055 mol), dissolved in 160 ml of water, 5 mol / L of potassium hydroxide or hydroxide at -5 ° C The pH of the sodium adjusted reaction system is weakly alkaline to pH 8, and the stirring reaction is 5 hours;
[0053]S2, the reactant of step S1 is concentrated, the concentrate is purified and methanol mixture, and the intermediate 1-methyl-2- (3-pyridine) -2-pyrrolidanol is obtained;
[0054]S3, dissolved in an intermediate with 180 ml of water and ethanol mixture, sodium borohydride (3.8 g) was added at -5 ° C, and the mixture was stirred to 15 ° C for 2 hours;
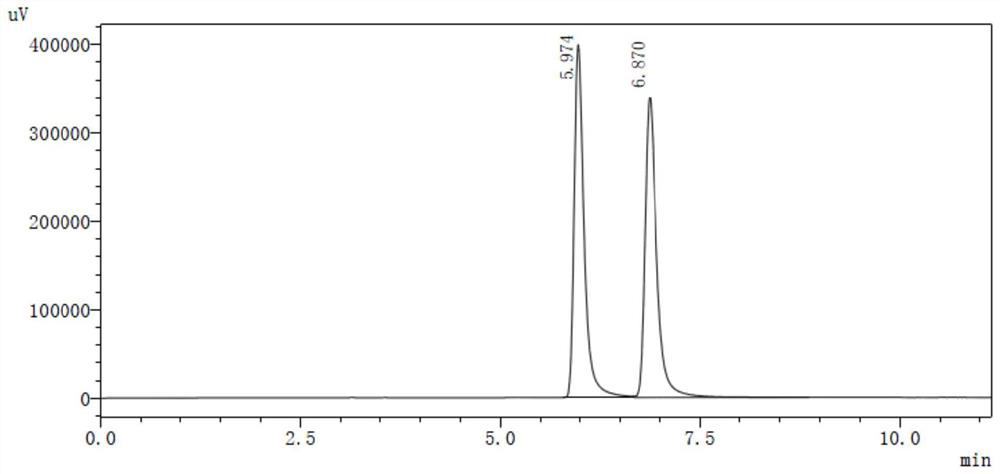
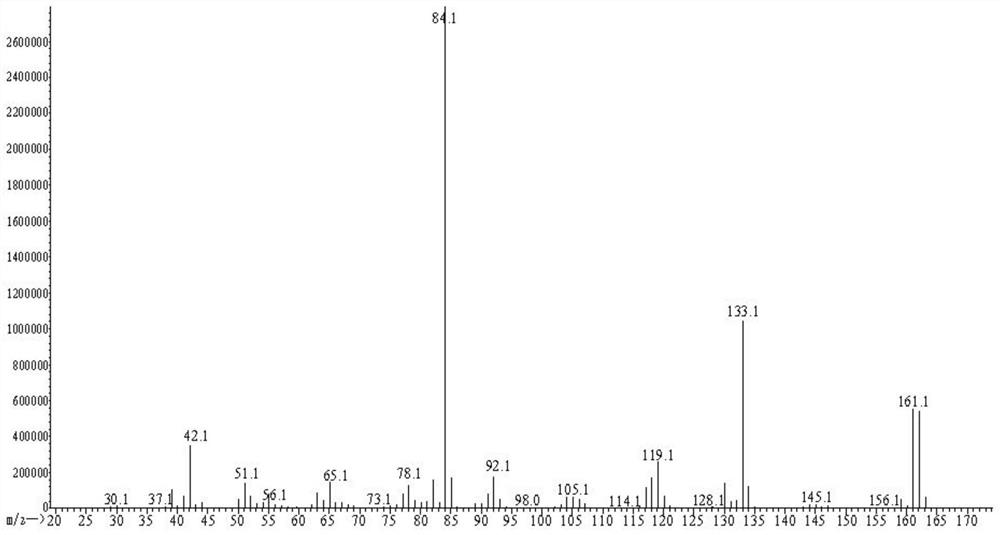
[0055]S4, extracted with ethyl acetate, dry concentrate on crude yellow oil, add 110 ml, directly evaporated, water is extracted with 270 ml of n-hexane or tetrahydrofuran, dry concentrate 7.9g, and test the hand with high performance liqu...
Embodiment 2
[0061]This embodiment provides an artificial synthesis (R, S)-nicotine salt, prepared according to the following steps:
[0062]S1, Take 4-methyl amino-1- (3-pyridine)-ketone hydrochloride (25.1 g, 0.1 mol), dissolved in 150 ml of water and methanol, sodium carbonate or potassium carbonate at 0 ° C to 8.5 , Stirring reaction for 3 hours;
[0063]S2, concentrate the reactants of step S1, concentrate water and ethanol mixture, to obtain intermediate 1-methyl-2- (3-pyridine) -2-pyrrolidanol;
[0064]S3, the intermediate body was dissolved with 160 ml of methanol, and lithium aluminum (3.7 g, 0.1 mol) was added at 0 ° C, warmed to 25 ° C, stirred for 2 hours;
[0065]S4, with methanol extraction, dry concentrate on crude yellow oil, 210 ml of water, directly evaporated, water was extracted with 290 ml of petroleum ether or diethyl ether, dried to concentrate 12.5 g, and the rotation of rotation is zero, that is (R) , S) - nicotine. HPLC test (R, S) - nicotine has a purity of 99.5%. The oxalic acid ...
Embodiment 3
[0067]This embodiment provides an artificial synthesis (R, S)-nicotine salt, prepared according to the following steps:
[0068]S1,4-methyl amino-1- (3-pyridine)-ketone hydrochloride (25.1 g, 0.1 mol), dissolved with 165 ml of water and ethanol, 5 ° C with sodium bicarbonate or potassium hydrogencarbonate pH to 7.5 , Stir for 2 hours;
[0069]S2, concentrate the reactant of step S1, concentrate water and methanol mixture, to obtain intermediate 1-methyl-2- (3-pyridine) -2-pyrrolidanol;
[0070]S3, the intermediate body was dissolved with 180 mL of propanol or isopropyl alcohol, and potassium borohydride (5.4 g, 0.1 mol) was added at 5 ° C, and the temperature was tapered to 25 ° C, and the mixture was stirred for 2 hours;
[0071]S4, 200 ml of distilled water, evaporation, evaporated water phase, extract the evaporated water phase was extracted with ethyl acetate, and ethyl acetate was concentrated to dryness 12.0 g, and the angiographic gauge is zero, that is, (R, S) - nicotine. HPLC test (R, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com