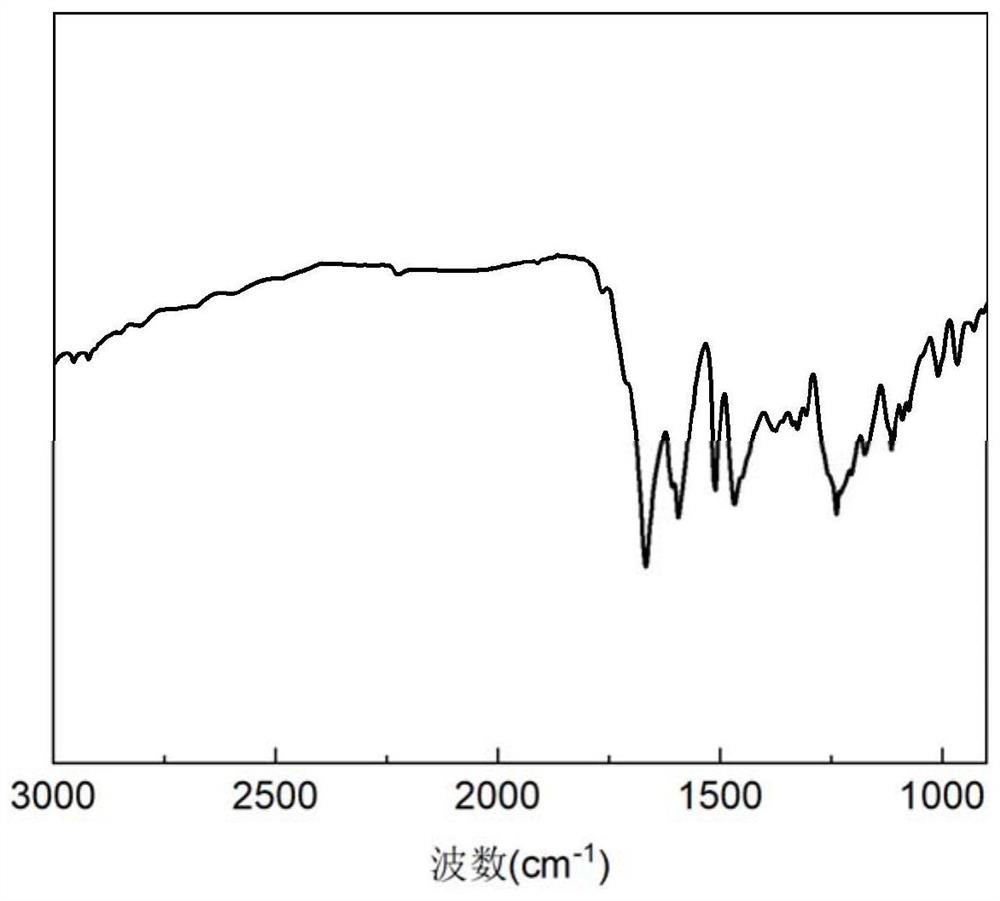
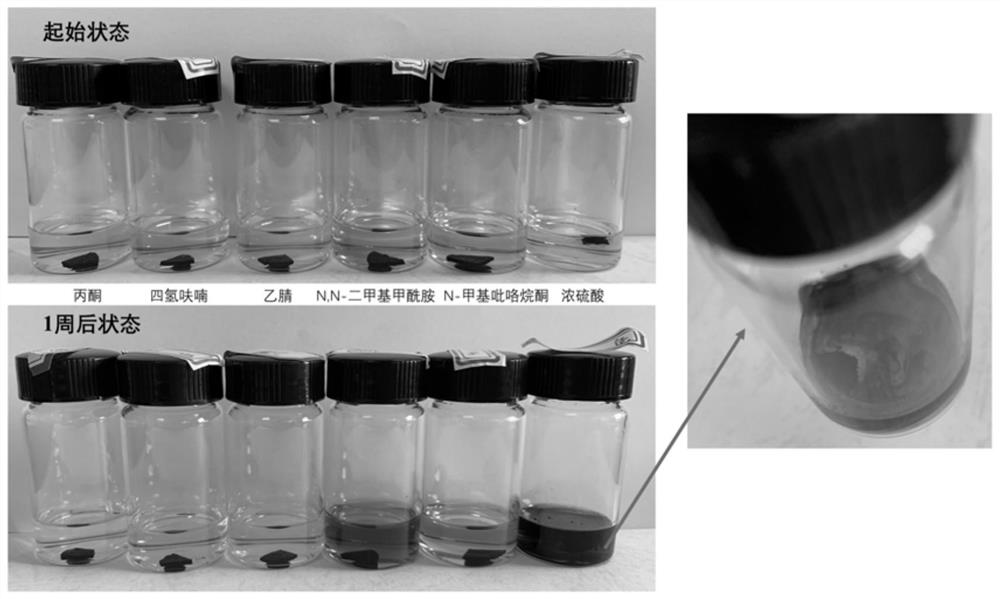
Phthalonitrile and amino acid cyclic peptide copolymer resin and preparation method thereof
A technology of cyclic peptide copolymerization resin and phthalonitrile, which is applied in the field of bio-based thermosetting resins, can solve the problems of low high temperature resistance, long time consumption, and a large number of bubbles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050]For the preparation method of phthalonitrile monomer with structural formula I-1, see Keller TM, Dominguez DD. High temperature resorcinol-based phthalonitrile polymer [J]. Polymer, 2005, 46(13): 4614-4618.
[0051] For the preparation method of phthalonitrile monomers with structural formulas I-2~I-4, see Liao S, Wu H, He X, etal. Promoting effect of methylne / methylene moiety of bisphenol E / F onphthalonitrile resin curing: Expanding the Structural design route of phthalonitrile resin [J]. Polymer, 2020, 210: 123001.
[0052] For the preparation method of phthalonitrile monomer with structural formula I-5, see Peng W, Yao F, Hu J, et al. Renewable protein-based monomer for thermosets: a case study on phthalonitrile resin [J]. Green Chemistry, 2018, 20(22):5158-5168.
[0053] The preparation method of the phthalonitrile monomer whose structural formula is I-6 refers to Keller T M. Synthesis and polymerization of multiple aromatic ether phthalonitriles [J]. Chemistry of m...
Embodiment 1
[0058] In this example, a copolymer resin of phthalonitrile and amino acid cyclic peptide was prepared by using resorcinol-type bis-phthalonitrile monomer and tyrosine cyclic dipeptide as raw materials, and resorcinol-type bis-phthalonitrile The chemical structural formula of forminonitrile monomer and tyrosine cyclic dipeptide is as follows:
[0059] Resorcinol type bisphthalonitrile monomer:
[0060] Tyrosine cyclic dipeptide:
[0061] Phthalonitrile and amino acid cyclic peptide copolymerization resin in the present embodiment are obtained through the following steps:
[0062] The two raw materials, resorcinol-type diphthalonitrile monomer and tyrosine cyclic dipeptide, were added to a single-necked flask with a magnetic stirrer in a ratio of 3:7, and added 5 times The solvent N,N-dimethylformamide of the total mass of the two raw materials. After the dissolution was complete, the solution was stirred at room temperature for 12 hours, then the solution was removed by...
Embodiment 2
[0072] In this example, bisphenol A type diphthalonitrile monomer and alanine cyclopeptide are used as raw materials to prepare phthalonitrile and amino acid cyclopeptide copolymer resin, bisphenol A type bisphthalonitrile monomer Body, alanine cyclic peptide chemical structure formula is as follows:
[0073] Bisphenol A type bisphthalonitrile monomer:
[0074] Alanine cyclic peptide:
[0075] Phthalonitrile and amino acid cyclic peptide copolymerization resin in the present embodiment are obtained through the following steps:
[0076] Add the two raw materials bisphenol A-type diphthalonitrile monomer and alanine cyclopeptide into a single-necked flask with a magnetic stirrer at a ratio of 1:4, and add 5 times the amount of the two The solvent tetrahydrofuran of the total mass of raw materials. After the dissolution was complete, the solution was stirred at room temperature for 12 hours, then the solution was removed by rotary evaporation at 60°C, and the residue was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com