Method for treating heavy metal ions in water body
A heavy metal ion and treatment method technology, applied in the field of heavy metal ion treatment in water, can solve the problems of poor water solubility of polymers and little production significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
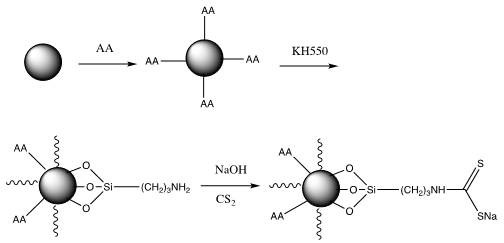
[0034] 1. Evenly disperse 3 g of ferric oxide microspheres with a particle size of 500 nm in 120 mL of deionized water, add 1.5 g of ascorbic acid, and heat to reflux for 4 hours. After the reaction, the product was separated with a strong magnet, washed three times with 20 mL of deionized water, and dried in vacuum to obtain ascorbic acid-modified Fe 3 o 4 @AA microspheres.
[0035] 2, 3g of the above-prepared Fe 3 o 4 The @AA microspheres were evenly dispersed in 150 mL of absolute ethanol, vacuumed and filled with nitrogen three times, and 1.5 g of silane coupling agent KH550 was added, stirred evenly at room temperature, and then refluxed for 2 hours under nitrogen protection. After the reaction, the product was separated with a high-strength magnet, washed three times with 20 mL of absolute ethanol, and dried in vacuum to obtain Fe 3 o 4 @AAKH550 microspheres.
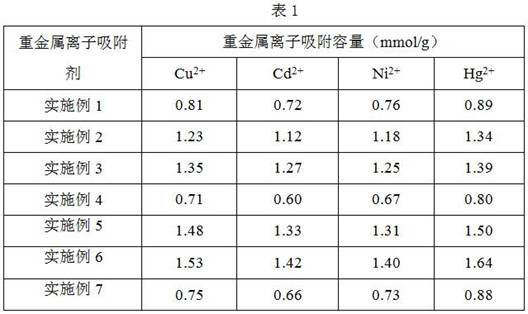
[0036] 3. Add 2gFe 3 o 4 @AAKH550 microspheres were dispersed in 20 mL of 1 mol / L NaOH aqueous solution, ...
Embodiment 2
[0038] 1. Evenly disperse 2 grams of ferric oxide microspheres with a particle size of 100 nm in 150 mL of deionized water, add 2 grams of ascorbic acid, and heat to reflux for 5 hours. After the reaction, the product was separated with a strong magnet, washed three times with 20 mL of deionized water, and dried in vacuum to obtain ascorbic acid-modified Fe 3 o 4 @AA.
[0039] 2, 2 grams of prepared Fe 3 o 4 The @AA microspheres were uniformly dispersed in 120 mL of absolute ethanol, vacuumed and filled with nitrogen three times, and 2 g of silane coupling agent KH550 was added, stirred evenly at room temperature, and then refluxed for 2 hours under the protection of nitrogen. After the reaction, the product was separated with a high-strength magnet, washed three times with 20 mL of ethanol, and dried in vacuum to obtain Fe 3 o 4 @AAKH550.
[0040] 3. Add 2 grams of Fe 3 o 4 @AAKH550 microspheres were dispersed in 7mL of 1 mol / L NaOH aqueous solution, and 1g of carbon ...
Embodiment 3
[0042] 1. Evenly disperse 2 grams of ferric oxide microspheres with a particle size of 100 nm in 150 mL of deionized water, add 2 grams of ascorbic acid, and heat to reflux for 3 hours. After the reaction, the product was separated with a strong magnet, washed three times with 20 mL of deionized water, and dried in vacuum to obtain ascorbic acid-modified Fe 3 o 4 @AA.
[0043] 2, 2 grams of prepared Fe 3 o 4 The @AA microspheres were evenly dispersed in 120 mL of absolute ethanol, vacuumed and filled with nitrogen three times, added 2 g of silane coupling agent KH550, stirred evenly at room temperature, and refluxed for 5 hours under nitrogen protection. After the reaction, the product was separated with a high-strength magnet, washed three times with 20 mL of ethanol, and dried in vacuum to obtain Fe 3 o 4 @AAKH550.
[0044] 3. Add 2 grams of Fe 3 o 4 @AAKH550 microspheres were dispersed in 5 mL of 4mol / L NaOH aqueous solution, and 2 g of carbon disulfide was slowly a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com