Nasal washing salt capable of providing nitric oxide and application thereof
A technology of nitric oxide and nasal washing, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, glycosylase, etc. Prominent and other problems, to achieve good efficacy and stability, inhibit bacterial growth, and improve the effect of rhinitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
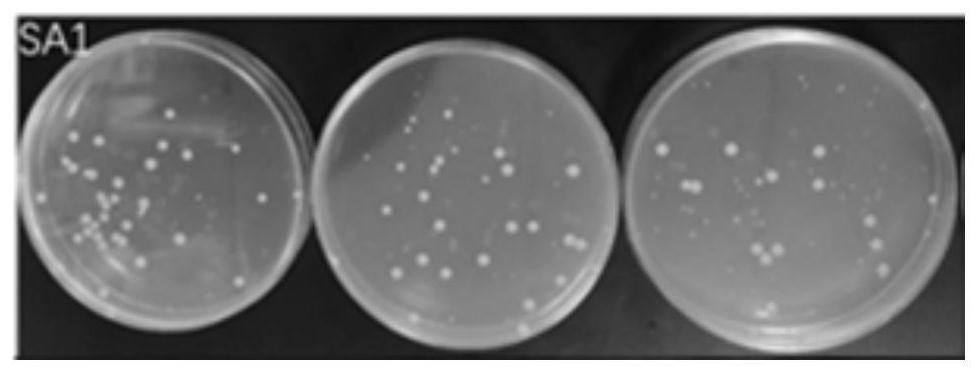
[0059] This embodiment provides a kind of nasal washing salt and nasal washing liquid that can provide nitric oxide by itself. The composition and content of its raw materials are as follows:
[0060] NO donor material (0.0075 part of S-nitrosoglutathione, 0.0025 part of arginine), 0.9 part of osmotic pressure regulator (NaCl), catalyst (0.01 part of glutathione, 0.01 part of ascorbic acid), pH adjustment Agent (0.08 part of citric acid), antibacterial agent (0.05 part of sodium benzoate. After mixing the above-mentioned raw materials at 20° C. to obtain the nasal wash salt.
[0061] NO donor material (S-nitrosoglutathione 0.0075%, arginine 0.0025%), osmotic pressure regulator (NaCl) 0.9%, catalyst (glutathione 0.01%, ascorbic acid 0.01%), pH adjustment agent (citric acid 0.08%), antibacterial agent (sodium benzoate 0.05%), and the balance of purified water. The NO donor material, osmotic pressure regulator, catalyst, pH regulator, bacteriostatic agent and purified water were...
Embodiment 2
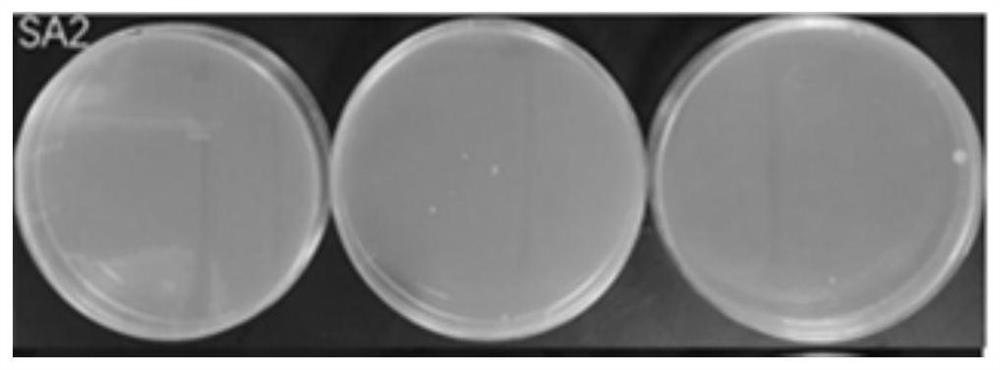
[0063] This embodiment provides a kind of nasal washing salt and nasal washing liquid that can provide nitric oxide by itself. The composition and content of its raw materials are as follows:
[0064] NO donor material (0.01 part of S-nitrosoglutathione, 0.005 part of arginine), 0.9 part of osmotic pressure regulator (NaCl), catalyst (0.005 part of glutathione, 0.01 part of ascorbic acid), pH adjustment agent (sodium citrate 0.2 parts), antibacterial agent (chitosan quaternary ammonium salt 0.1 part). The above-mentioned raw materials were mixed uniformly at 20° C. to obtain the nasal washing salt.
[0065] NO donor material (S-nitrosoglutathione 0.01%, arginine 0.005%), osmotic pressure regulator (NaCl) 0.9%, catalyst (glutathione 0.005%, ascorbic acid 0.01%), pH adjustment Agent (sodium citrate 0.2%), antibacterial agent (chitosan quaternary ammonium salt 0.1%), purified water balance. The NO donor material, osmotic pressure regulator, catalyst, pH regulator, bacteriostati...
Embodiment 3
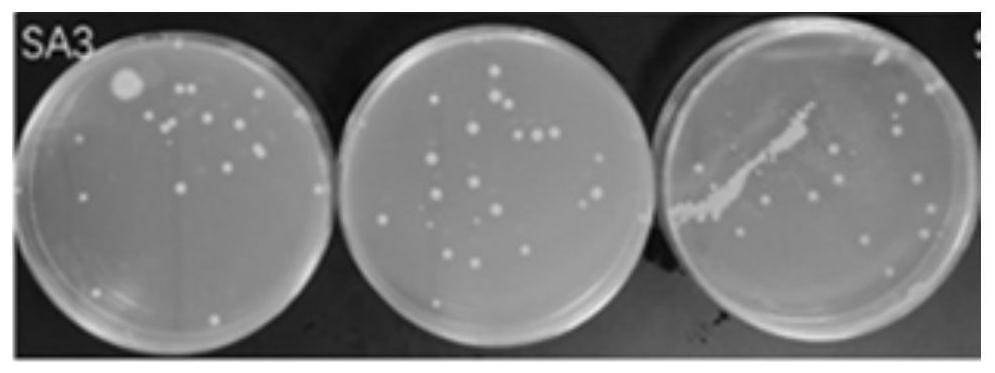
[0067] This embodiment provides a kind of nasal washing salt and nasal washing liquid that can provide nitric oxide by itself. The composition and content of its raw materials are as follows:
[0068] NO donor material (0.008 part of S-nitrosoglutathione, 0.002 part of arginine), 0.9 part of osmotic pressure regulator (NaCl), catalyst (0.01 part of glutathione, 0.005 part of ascorbic acid), pH adjustment agent (0.1 part of disodium hydrogen phosphate), antibacterial agent (0.1 part of dodecyltrimethylammonium bromide). The above-mentioned raw materials were mixed uniformly at 20° C. to obtain the nasal washing salt.
[0069] NO donor material (S-nitrosoglutathione 0.008%, arginine 0.002%), osmotic pressure regulator (NaCl) 0.9%, catalyst (glutathione 0.01%, ascorbic acid 0.005%), pH adjustment agent (disodium hydrogen phosphate 0.1%), antibacterial agent (dodecyltrimethylammonium bromide 0.1%), and the balance of purified water. The NO donor material, osmotic pressure regula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com