Octanoic acid acylation modified antibacterial peptide and application thereof
An antibacterial peptide and amino acid technology, applied in the fields of genetic engineering and biological preparations, can solve the problems of poor activity, insufficient antibacterial activity of fungi, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
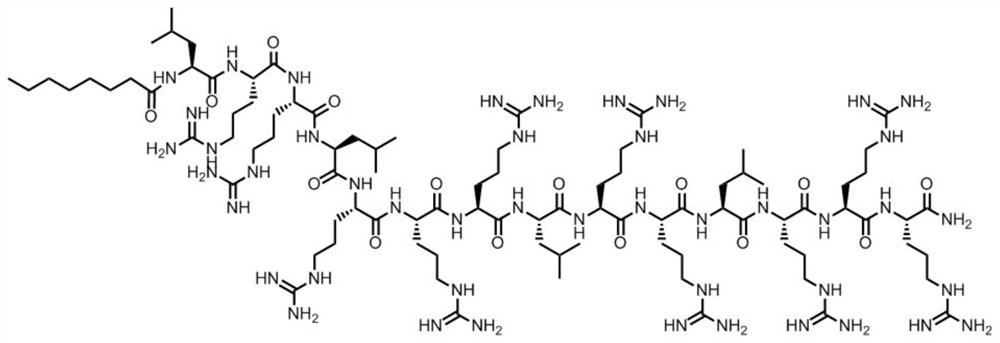
[0028] In this embodiment, the antimicrobial peptide of the present invention (see SEQ ID NO.1) is synthesized by solid-phase chemical synthesis, and the peptide structure is as follows: figure 1 Shown:
[0029] 1. The preparation of antimicrobial peptides is carried out one by one from the C-terminal to the N-terminal, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed chain protection resin;
[0030] 2. Add a cleavage reagent to the peptide resin obtained above, react for 2 hours at 20°C in the dark, filter; wash the precipitate wi...
Embodiment 2
[0034] Determination of the antifungal activity of the peptide: the minimum inhibitory concentration of the peptide (C8LR) prepared in Example 1 against fungi was determined by microdilution method. Add 0.2% fetal bovine serum albumin containing 0.01% acetic acid as a diluent into a 96-well plate, and use the serial dilution method to configure the antibacterial peptide solution obtained in Example 1 of the present invention in sequence, so that the solution in each well is The volume is 50 μL. Then add 50 μL of the bacteria solution to be tested (~10 3 CFU / mL) in each well, the medium is RPMI 1640 (pH=7.0) containing MOPS. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 28°C for 48 hours, then measure the light absorption value at 492nm with a microplate reader, and use the value greater than 0.1 as the criterion for the growth of the...
Embodiment 3
[0039] Determination of the antibacterial activity of the peptide: the minimum inhibitory concentration of the peptide (C8LR) prepared in Example 1 against bacteria was determined by microdilution method. Add 0.2% fetal bovine serum albumin containing 0.01% acetic acid as a diluent into a 96-well plate, and sequentially prepare a series of gradient antimicrobial peptide solutions using the doubling dilution method, so that the volume of the solution in each well is 50 μL. Then add 50 μL of the bacteria solution to be tested (~10 5 CFU / mL) in each well, the medium is MHB (pH=7.0). Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 37°C for 18 hours, then measure the light absorption value at 492nm with a microplate reader, and use the value greater than 0.1 as the judgment standard for the growth of the strain to determine the minimum inhib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com